QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
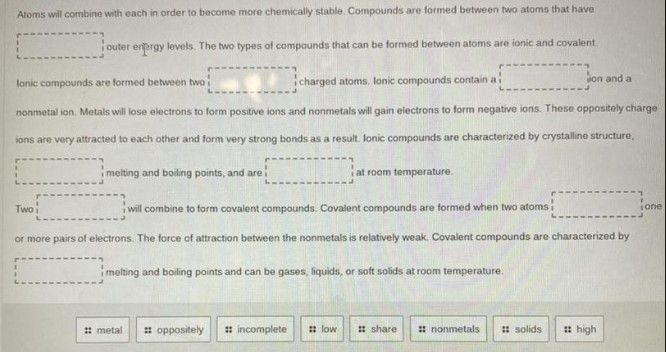
Atoms will combine with each in order to become more chemically stable. Compounds are formed between two atoms that have
| | |
| --- | --- |
| | |
outer energy levels. The two types of compounds that can be formed between atoms are ionic and covalent.
Ionic compounds are formed between two charged atoms. Ionic compounds contain a ion and a nonmetal ion. Metals will lose electrons to form positive ions and nonmetals will gain electrons to form negative ions. These oppositely charge ions are very attracted to each other and form very strong bonds as a result. Ionic compounds are characterized by crystalline structure,
| | |
| --- | --- |
| | |
melting and boiling points, and are at room temperature.
Two will combine to form covalent compounds. Covalent compounds are formed when two atoms
| | |
| --- | --- |
| | |
one or more pairs of electrons. The force of attraction between the nonmetals is relatively weak. Covalent compounds are characterized by
| | |
| --- | --- |
| | |
melting and boiling points and can be gases, liquids, or soft solids at room temperature.
| | |
| --- | --- |
| | |
metal | oppositely | incomplete | low | share | nonmetals | solids | high
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Define an ionic compound
An ionic compound is formed between two charged atoms, consisting of a metal ion and a nonmetal ion. Metals lose electrons to form positive ions, while nonmetals gain electrons to form negative ions. These oppositely charged ions are attracted to each other, creating strong bonds and resulting in crystalline structure, high melting and boiling points, and solid form at room temperature.
Step 2: Define a covalent compound
A covalent compound is formed when two atoms share one or more pairs of electrons. This force of attraction between nonmetals is relatively weak compared to ionic compounds. Covalent compounds are characterized by lower melting and boiling points and can exist as gases, liquids, or soft solids at room temperature.
Final Answer
Ionic compounds are formed between metal and nonmetal ions through the transfer of electrons, resulting in strong bonds, crystalline structure, and high melting and boiling points. Covalent compounds are formed when two atoms share electrons, creating relatively weak bonds and existing in various physical states at room temperature.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students