QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
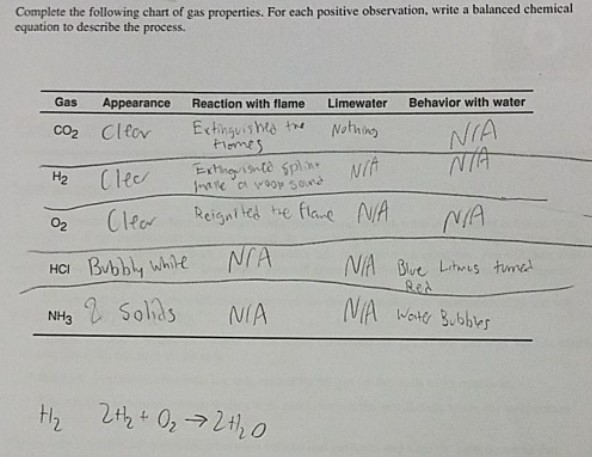
Complete the following chart of gas properties. For each positive observation, write a balanced chemical equation to describe the process.
| Gas | Appearance | Reaction with flame | Limewater | Behavior with water |
| --- | --- | --- | --- | --- |
| CO^2 | Clear | Extinguishes the flames | Nothing | N/A |
| H^2 | Clear | Extinguishes spills, Holes or vapor gases | N/A | N/A |
| O^2 | Clear | Reignites the flame | N/A | N/A |
| HCl | Bubbly white | N/A | N/A | Blue Litmus turned red |
| NH^3 | Sodids | N/A | N/A | Water Bubbles |
H_2 \quad 2H_2 + O_2 \rightarrow 2H_2 O
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Write the balanced chemical equation for the reaction of hydrogen gas (H2) with oxygen gas (O2) to produce water (H^2O).
2H_2 + O_2 \rightarrow 2H_2O
Step 2: Explain the reaction.
Hydrogen gas (H2) reacts with oxygen gas (O2) in a combustion reaction to produce water (H^2O). This reaction is highly exothermic, releasing a large amount of energy in the form of heat and light.
Final Answer
The balanced chemical equation for the reaction of hydrogen gas (H2) with oxygen gas (O2) to produce water (H^2O) is: 2H_2 + O_2 \rightarrow 2H_2 O
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students