QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
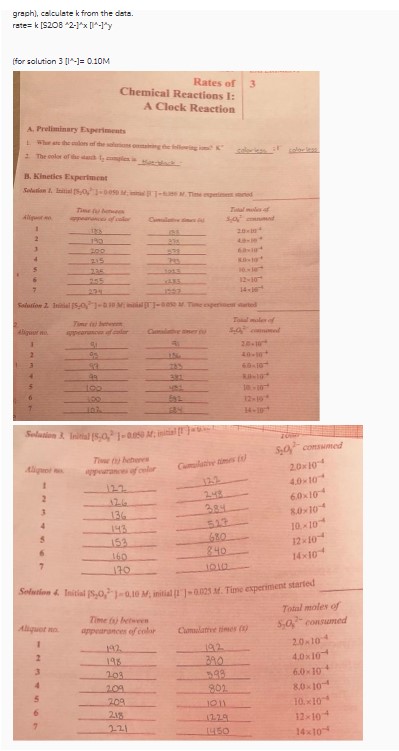
graph), calculate k from the data. rate= k [S^2O^5 * 2 -|* x |*|*|*|*|
| (for solution 2 | | | 3 |
| --- | --- | --- | --- |
| | | | |
| Chemical Reactions 1: | | | |
| A Clock Reaction | | | |
| A. Preliminary Experiments | | | |
| 1. | | | |
| 2. | | | |
| 3. | | | |
| 4. | | | |
| B. Kinetics Experiment | | | |
| Solution 1. Initial [S^2O^5 * 2 -|* x 0.05 M; initial [1 1 - 0.005 M. Time experiment started | | | |
| | | | |
| Aliquot no. | Time (s) between | | Total moles of |
| | appearances of color | Cumulative times (s) | S^2O^5 * consumed |
| 1 | 18.5 | 18.5 | 2.0×10 - 4 |
| 2 | 19.0 | 27.0 | 4.0×10 - 4 |
| 3 | 20.0 | 29.0 | 6.0×10 - 4 |
| 4 | 21.5 | 31.5 | 8.0×10 - 4 |
| 5 | 22.0 | 32.0 | 10×10 - 4 |
| 6 | 23.5 | 33.5 | 12×10 - 4 |
| 7 | 24.5 | 35.0 | 14×10 - 4 |
| | | | |
| Solution 2. Initial [S^2O^5 * 2 - 0.10 M; initial [1 1 - 0.010 M. Time experiment started | | | |
| | | | |
| Aliquot no. | Time (s) between | | Total moles of |
| | appearances of color | Cumulative times (s) | S^2O^5 * consumed |
| 1 | 4.0 | 4.0 | 2.0×10 - 4 |
| 2 | 4.5 | 5.0 | 4.0×10 - 4 |
| 3 | 5.5 | 6.0 | 6.0×10 - 4 |
| 4 | 6.5 | 7.0 | 8.0×10 - 4 |
| 5 | 7.0 | 8.0 | 10×10 - 4 |
| 6 | 8.0 | 9.0 | 10×10 - 4 |
| 7 | 9.0 | 10.0 | 12×10 - 4 |
| | | | |
| Solution 3. Initial [S^2O^5 * 2 - 0.05 M; initial [1 1 - 0.010 M. Time experiment started | | | |
| | | | |
| Aliquot no. | Time (s) between | | Total moles of |
| | appearances of color | Cumulative times (s) | S^2O^5 * consumed |
| 1 | 13.5 | 13.5 | 4.0×10 - 4 |
| 2 | 13.0 | 14.0 | 6.0×10 - 4 |
| 3 | 13.5 | 14.5 | 8.0×10 - 4 |
| 4 | 14.5 | 15.0 | 10×10 - 4 |
| 5 | 15.5 | 16.0 | 12×10 - 4 |
| 6 | 16.0 | 17.0 | 14×10 - 4 |
| 7 | 17.0 | 18.0 | 14×10 - 4 |
| | | | |
| Solution 4. Initial [S^2O^5 * 2 - 0.10 M; initial [1 1 - 0.025 M. Time experiment started | | | |
| | | | |
| Aliquot no. | Time (s) between | | Total moles of |
| | appearances of color | Cumulative times (s) | S^2O^5 * consumed |
| 1 | 19.5 | 19.5 | 2.0×10 - 4 |
| 2 | 19.5 | 20.0 | 4.0×10 - 4 |
| 3 | 20.5 | 21.5 | 6.0×10 - 4 |
| 4 | 21.5 | 22.0 | 8.0×10 - 4 |
| 5 | 22.0 | 23.0 | 10×10 - 4 |
| 6 | 23.5 | 24.5 | 12×10 - 4 |
| 7 | 25.0 | 25.5 | 14×10 - 4 |
| | | | |
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I'll solve this kinetics problem by calculating the rate constant k for each solution using the integrated rate law method.
I'll focus on Solution 2 as an example.
Step 2: Identify the Reaction Order
- Rate law appears to be: $$rate = k[S_{2}O_{5}^{2-}]^{x}[I^{-}]^{y}
- The problem involves determining the rate law and rate constant
Final Answer
- k = slope / ([A]_{0}^{n- 1}), where n is reaction order
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students