QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
# Module 6 Test
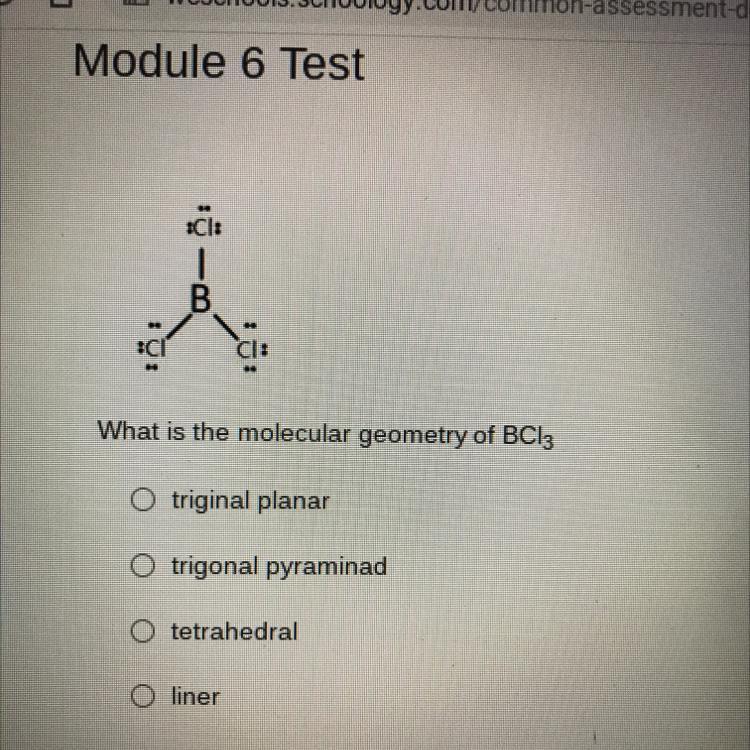
What is the molecular geometry of $\mathrm{BCl}_{3}$
$\bigcirc$ triginal planar
$\bigcirc$ trigonal pyramid
$\bigcirc$ tetrahedral
$\bigcirc$ liner
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I'll solve this step by step using VSEPR theory to determine the molecular geometry of \mathrm{BCl}_{3}.
Step 2: Determine the central atom and number of valence electrons
- Central atom is Boron (B) - Boron has 3 valence electrons - Each Chlorine (Cl) contributes 7 valence electrons
Final Answer
The correct answer is trigonal planar, which matches the first option in the original multiple-choice question.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students