QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
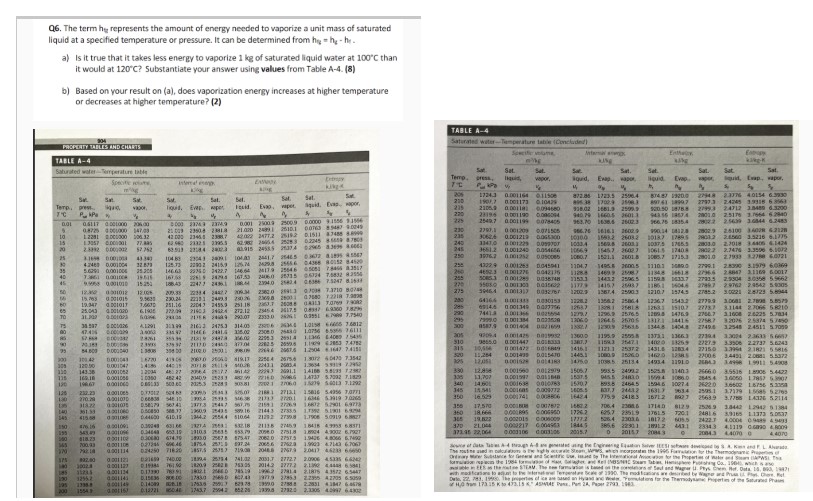
Q^6. The term h₀ represents the amount of energy needed to vaporize a unit mass of saturated liquid at a specified temperature or pressure. It can be determined from h₀ = h₀ - h₁.
a) Is it true that it takes less energy to vaporize 1 kg of saturated liquid water at 100°C than it would at 120°C? Substantiate your answer using **values** from Table A- 4. **(8)**
b) Based on your result on (a), does vaporization energy increase at higher temperature or decreases at higher temperature? **(2)**
| TABLE A- 4 | | | | | | | | | | | | | | | | | | | |
| --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I apologize, but the table you referenced is not fully visible in the image.
Without the specific values from Table A- 4, I cannot complete the precise calculations. However, I can guide you through the problem-solving approach:
Step 2: Understanding the Problem
- The problem asks about the energy ($$h_{0}$$) needed to vaporize 1 kg of saturated liquid water at different temperatures
- We need to compare the vaporization energy at 100°C and 120°C
Final Answer
Compare the values to determine which requires more energy Without the table values, I cannot provide the definitive solution. Would you be able to share the specific values from Table A- 4?
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students