QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
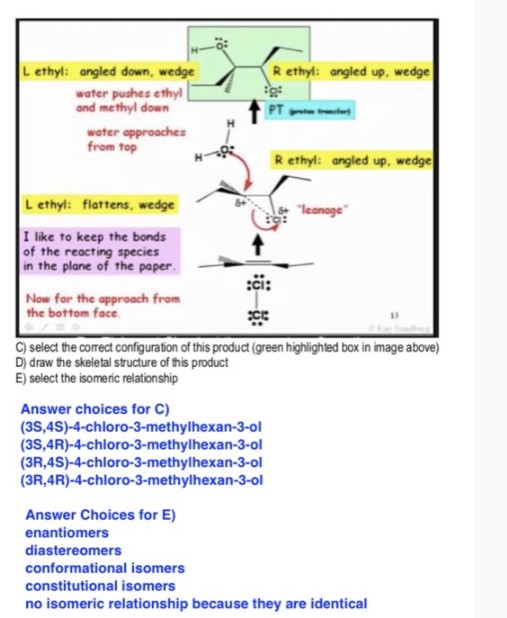
C) select the correct configuration of this product (green highlighted box in image above)
D) draw the skeletal structure of this product
E) select the isomeric relationship
Answer choices for C)
(3S,4S)- 4 -chloro- 3 -methylhexan- 3 -ol
(3S,4R)- 4 -chloro- 3 -methylhexan- 3 -ol
(3R,4S)- 4 -chloro- 3 -methylhexan- 3 -ol
(3R,4R)- 4 -chloro- 3 -methylhexan- 3 -ol
Answer Choices for E)
enantiomers
diastereomers
conformational isomers
constitutional isomers
no isomeric relationship because they are identical
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I'll solve this organic chemistry problem step by step, focusing on stereochemistry and isomeric relationships.
Step 2: Analyze the Molecular Structure
- The molecule is 4 -chloro- 3 -methylhexan- 3 -ol - It contains two chiral centers (carbon atoms with four different substituents) - The stereochemistry of these centers determines the specific configuration
Final Answer
C) (3R,4S)- 4 -chloro- 3 -methylhexan- 3 -ol E) diastereomers Note: This solution requires understanding stereochemical principles and spatial arrangement of molecular groups.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students