QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
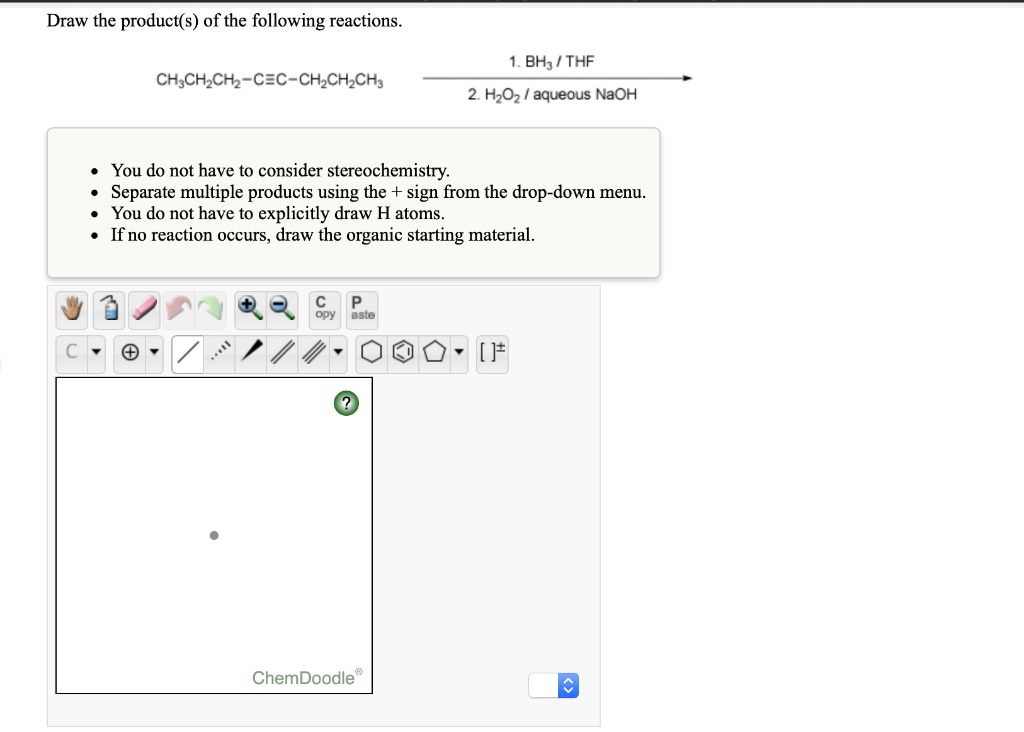
Draw the product(s) of the following reactions.
\begin{aligned}
& \text { 1. } \mathrm{BH}_{3} / \mathrm{THF} \\
& \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2}=\mathrm{C} \equiv \mathrm{C}-\mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{3}
\end{aligned}
- You do not have to consider stereochemistry.
- Separate multiple products using the + sign from the drop-down menu.
- You do not have to explicitly draw H atoms.
- If no reaction occurs, draw the organic starting material.
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the reagents and their roles.
In this reaction, $\text{BH}_{3}/\text{THF}$ is acting as a reducing agent, and the alkyne $\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}=\text{C}\equiv\text{C}-\text{CH}_{2}\text{CH}_{2}\text{CH}_{3}$ is the organic compound being reduced.
Step 2: Understand the reduction process.
Borane ($\text{BH}_{3}$) in tetrahydrofuran (THF) is a strong reducing agent, which can selectively reduce alkynes to trans-alkenes (also known as cis-alkenes).
The reduction process involves the addition of two hydrogen atoms across the triple bond, resulting in a carbon-carbon double bond.
Final Answer
The product of the reaction between $\text{BH}_{3}/\text{THF}$ and the alkyne $\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}=\text{C}\equiv\text{C}-\text{CH}_{2}\text{CH}_{2}\text{CH}_{3}$ is: \text{CH}_{3}\text{CH}_{2}\text{CH}_{2}-\text{CH}=\text{CH}-\text{CH}_{2}\text{CH}_{2}\text{CH}_{3}
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students