QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
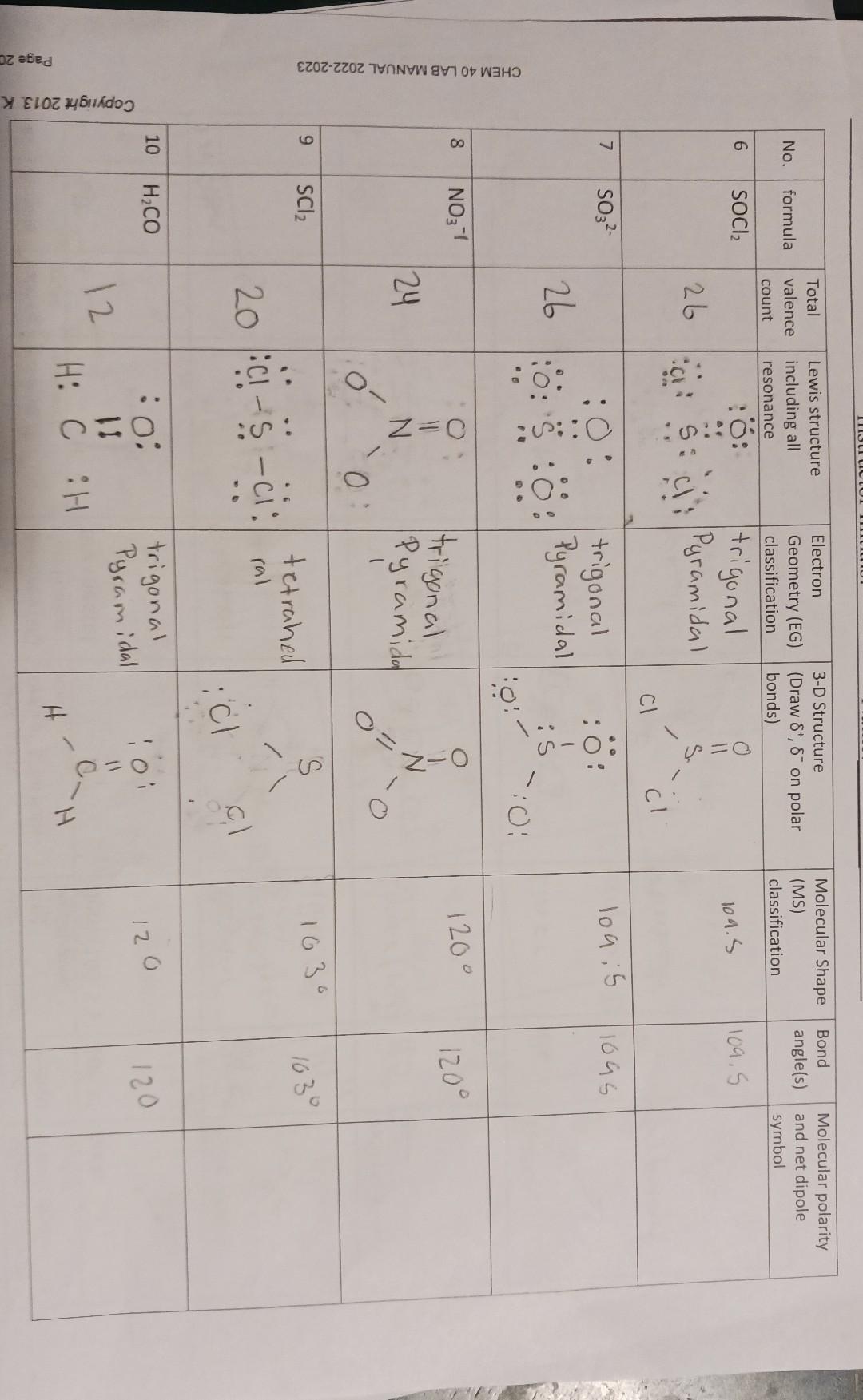
| No. | formula | Total valence count | Lewis structure including all resonance | Electron Geometry (EG) classification | 3 -D Structure (Draw B', B' on polar bonds) | Molecular Shape (MS) classification | Bond angle(s) | Molecular polarity and net dipole symbol |
| --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 6 | 30Cl | 26 | :0: 5: 0: 5: 0: | trigonal Pyramidal | 0 11 5 0 0 0 | 104.5 | 104.5 | |
| 7 | 30s² | 26 | :0: 5: 0: 5: 0: | trigonal Pyramidal | :0: 5: 0: 0: 0 | 104.5 | 104.5 | |
| 8 | NO₃⁻⁷ | 24 | 0 1 0: 0: 0: 0: | trigonal Pyramidal | 0 1 0: 0: 0: 0 | 120° | 120° | |
| 9 | 30L | 20 | :0: - 5: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0: - 0
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1**Problem:** Given the information in the table, determine the molecular shape and bond angle(s) for each molecule or ion.
Step 2** 6: 30Cl** - Total valence count is 26.
The Lewis structure including all resonance is:Cl :0: Cl :5: Cl :0: Cl :5: 0:. The electron geometry classification is trigonal pyramidal, and there are no lone pairs on the central atom. The 3 -D structure is drawn with B' on polar bonds. The molecular shape classification is also trigonal pyramidal, and the bond angle is approximately 104.5 degrees. Since there is a positive charge on the central atom, the molecule is polar. **Molecular Shape:** Trigonal Pyramidal **Bond Angle:** ~104.5 degrees **Molecular Polarity:** Polar (Cl has a positive partial charge)
Final Answer
1. ** 6: 30Cl:** Molecular Shape: Trigonal Pyramidal, Bond Angle: ~104.5 degrees, Molecular Polarity: Polar (Cl has a positive partial charge) 2. ** 7: 30s²:** Molecular Shape: Trigonal Pyramidal, Bond Angle: ~104.5 degrees, Molecular Polarity: Polar (S has a negative partial charge) 3. ** 8: NO₃⁻⁷:** Molecular Shape: Trigonal Pyramidal, Bond Angle: ~120 degrees, Molecular Polarity: Nonpolar (the negative charge is distributed evenly among the oxygen atoms) 4. ** 9: 30L:** Molecular Shape: Linear, Bond Angle: 180 degrees, Molecular Polarity: Polar (L has a negative partial charge)
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students