QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
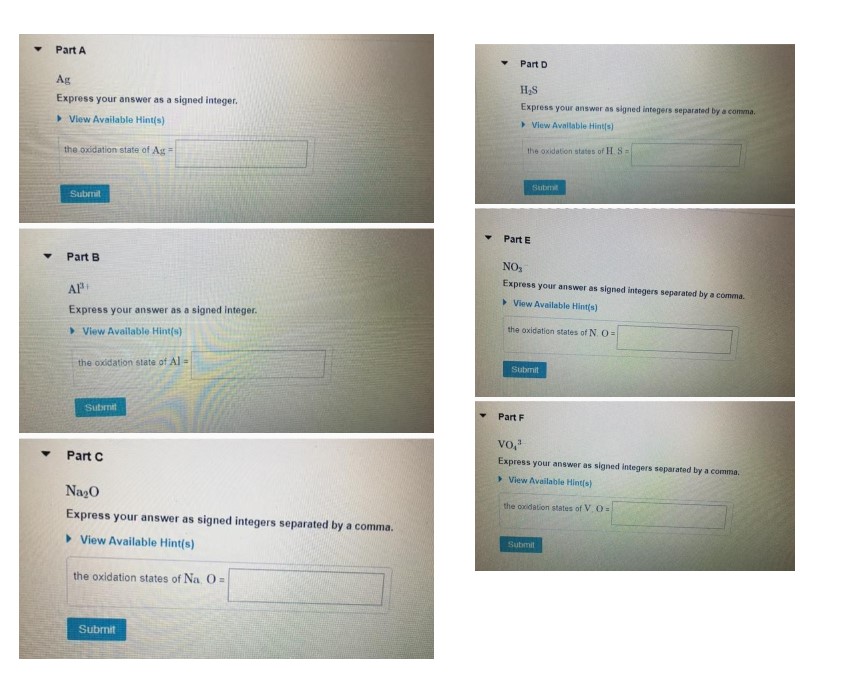
### Part A
**Ag**
Express your answer as a signed integer.
- **View Available Hint(s)**
the oxidation state of Ag =
Subtotal: 1
### Part B
**Al³**
Express your answer as a signed integer.
- **View Available Hint(s)**
the oxidation state of Al³ =
Subtotal: 1
### Part C
**Na₂O**
Express your answer as signed integers separated by a comma.
- **View Available Hint(s)**
the oxidation states of Na₂O =
Subtotal: 1
### Part D
**H₂S**
Express your answer as signed integers separated by a comma.
- **View Available Hint(s)**
the oxidation states of H₂S =
Subtotal: 1
### Part E
**NO₃**
Express your answer as signed integers separated by a comma.
- **View Available Hint(s)**
the oxidation states of NO₃ =
Subtotal: 1
### Part F
**VO₄³**
Express your answer as signed integers separated by a comma.
- **View Available Hint(s)**
the oxidation states of VO₄³ =
Subtotal: 1
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I'm here to help you understand how to find the oxidation states for each of the given compounds.
Let's go through this step-by-step.
Step 2**Ag**: Silver (Ag) is a transition metal, and its oxidation state is typically + 1.
So, the oxidation state of Ag is **+ 1 **.
Final Answer
Part A: + 1 Part B: + 3 Part C: + 1, + 1, - 2 Part D: + 1, + 1, - 2 Part E: + 5, - 2, - 2, - 2 Part F: + 5, - 2, - 2, - 2, - 2
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students