QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
# Question 26 of 64
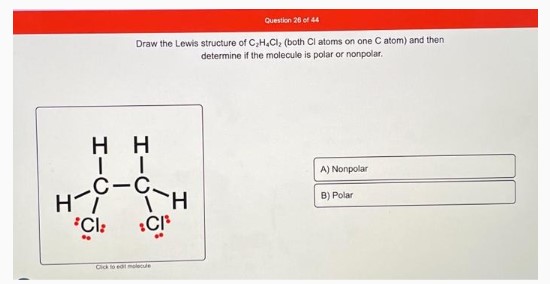
Draw the Lewis structure of C₃H₈Cl₂ (both Cl atoms on one C atom) and then determine if the molecule is polar or nonpolar.
| A) Nonpolar | |
| --- | --- |
| B) Polar | |
Click to edit molecule
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I'll solve this step by step using proper Lewis structure drawing and polarity analysis:
Step 2: Determine Total Valence Electrons
- Total valence electrons: $$12 + 8 + 14 = 34$$ electrons
- Carbon (C): 4 valence electrons × 3 carbons = 12 - Hydrogen (H): 1 valence electron × 8 hydrogens = 8 - Chlorine (Cl): 7 valence electrons × 2 chlorines = 14
Final Answer
The molecule \text{C}_3\text{H}_8\text{Cl}_2 is polar due to the uneven distribution of electronegative chlorine atoms on one carbon.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students