QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
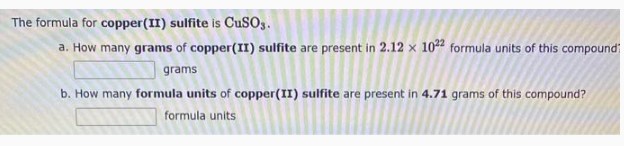
The formula for copper(II) sulfite is $\mathrm{CuSO}_{3}$.
a. How many grams of copper(II) sulfite are present in $2.12 \times 10^{22}$ formula units of this compound
$\square$ grams
b. How many formula units of copper(II) sulfite are present in 4.71 grams of this compound?
$\square$ formula units
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I'll solve this problem step by step using the specified LaTeX formatting guidelines.
Step 2: Calculate the molar mass of copper(II) sulfite \mathrm{CuSO}_{3}
\begin{align}
- Atomic masses: - Molar mass calculation: \mathrm{Molar~Mass} &= 63.55 + 32.07 + (3 \times 16.00) \ &= 63.55 + 32.07 + 48.00 \ &= 143.62 \mathrm{~g/mol} \end{align}
Final Answer
a. 5.05 \mathrm{~g} of copper(II) sulfite b. 1.98 \times 10^{22} \mathrm{~formula~units} of copper(II) sulfite
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students