QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
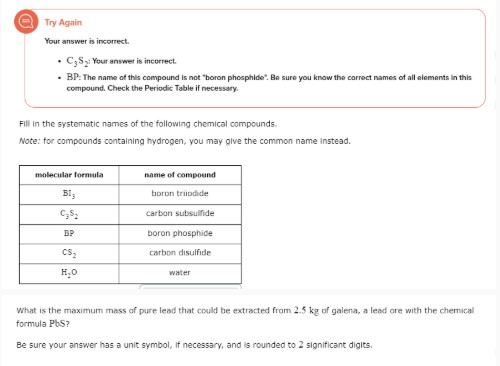
# Try Again
Your answer is incorrect.
- $\mathrm{C}*{2} \mathrm{~S}*{2}$ : Your answer is incorrect.
- BP: The name of this compound is not "boron phosphide". Be sure you know the correct names of all elements in this compound. Check the Periodic Table if necessary.
Fill in the systematic names of the following chemical compounds. Note: for compounds containing hydrogen, you may give the common name instead.
| molecular formula | name of compound |
| --- | --- |
| $\mathrm{Bl}*{1}$ | boron triiodide |
| $\mathrm{C}*{2} \mathrm{~S}*{2}$ | carbon subsulfide |
| BP | boron phosphide |
| $\mathrm{CS}_{2}$ | carbon disulfide |
| $\mathrm{H}_{2} \mathrm{O}$ | water |
What is the maximum mass of pure lead that could be extracted from 2.5 kg of galena, a lead ore with the chemical formula PbS ?
Be sure your answer has a unit symbol, if necessary, and is rounded to 2 significant digits.
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I'll solve this problem step by step, focusing on determining the maximum mass of lead that can be extracted from galena (PbS).
Step 2: Identify the given information
- Mass of galena = $$2.5 \times 10^{3} \mathrm{~g}$$ (2.5 kg converted to grams)
- Ore: Galena (PbS)
Final Answer
2.2 \times 10^{3} \mathrm{~g} or 2.2 \mathrm{~kg} of pure lead
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students