QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
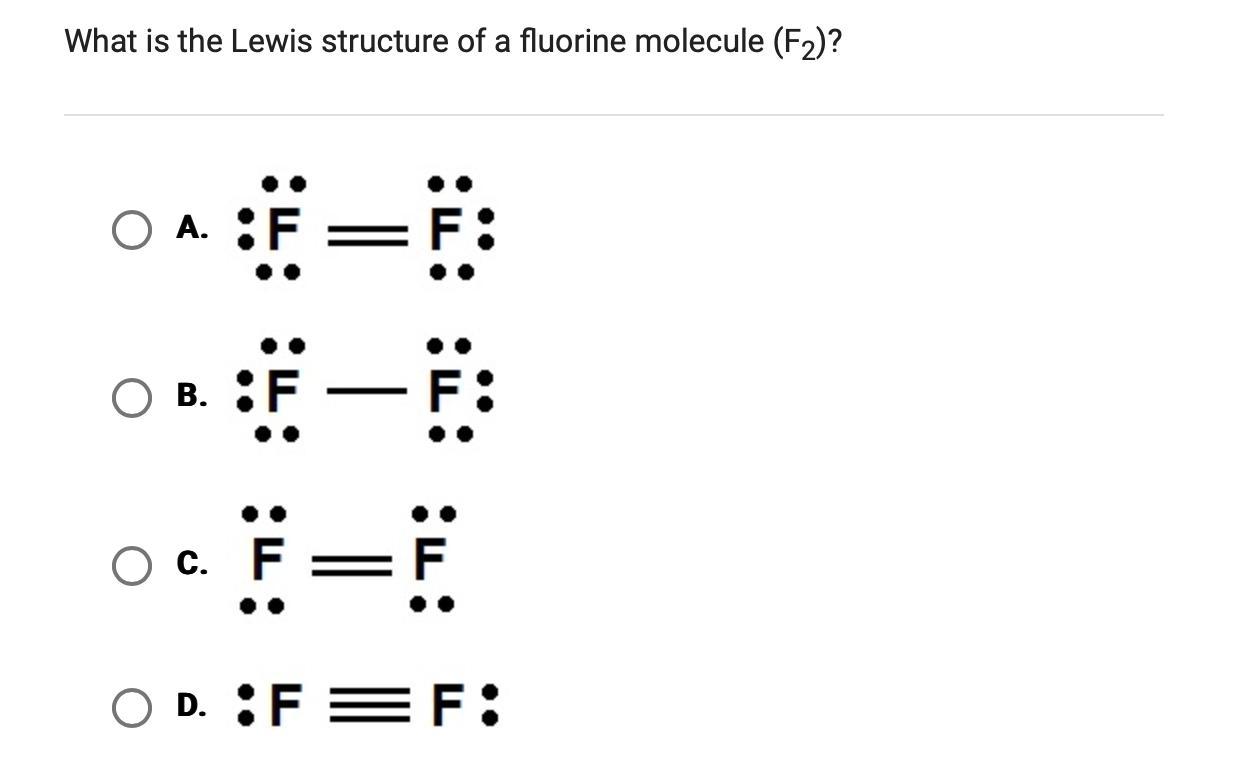
What is the Lewis structure of a fluorine molecule $\left(\mathrm{F}_{2}\right)$ ?
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Determine the total number of valence electrons in the F₂ molecule.
Fluorine is in the 17th group of the periodic table, so it has 7 valence electrons. In the F₂ molecule, there are 2 fluorine atoms. Therefore, the total number of valence electrons is:
Final Answer
Fluorine is in the 17th group of the periodic table, so it has 7 valence electrons. In the F₂ molecule, there are 2 fluorine atoms. Therefore, the total number of valence electrons is:
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students