QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
Which equation represents the combined gas law?
Attachments
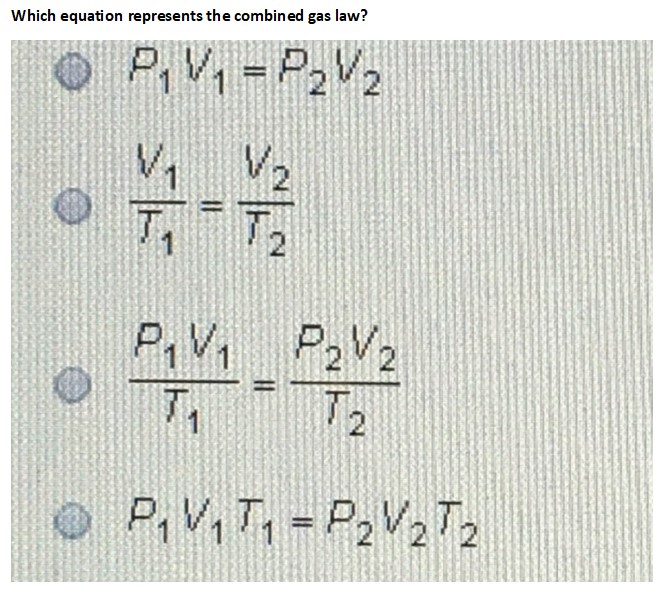
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: The combined gas law is a combination of three gas laws: Boyle's law, Charles' law, and Gay-Lussac's law.
\frac{P_1V_1}{T_1} = \frac{P_2V_2}{T_2}
The formula for the combined gas law is given by: where: - 1$ is the final temperature (in Kelvin)
Step 2: To use the combined gas law, you need to rearrange the formula to solve for the desired variable.
V_2 = \frac{P_2T_2V_1}{P_1T_1}
For example, if you want to find the final volume 1$, you can rearrange the formula as follows:
Final Answer
The combined gas law is represented by the formula \frac{P_1 V_1}{T_1} = \frac{P_2 V_2}{T_2}, where 1$ is the final temperature (in Kelvin).
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students