QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
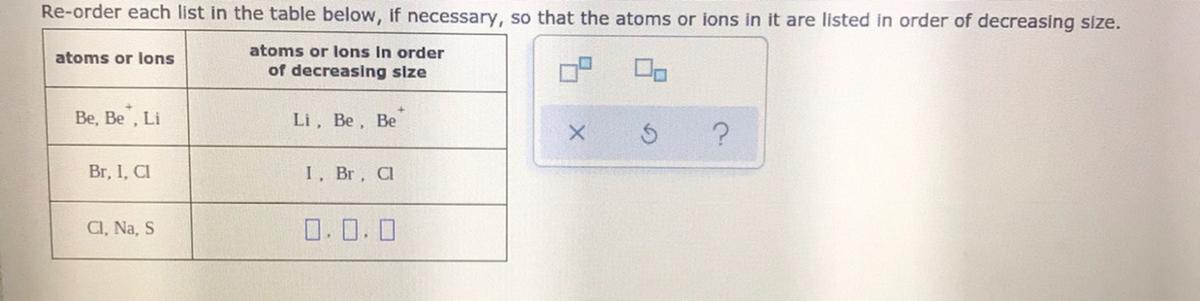
Re-order each list in the table below, if necessary, so that the atoms or ions in it are listed in order of decreasing size.
| atoms or ions | atoms or ions in order of decreasing size |
| --- | --- |
| Be, Be⁺, Li | Li, Be, Be⁺ |
| Br, I, Cl | I, Br, Cl |
| Cl, Na, S | ☐, ☐, ☐ |
☐ ☐ ☐
☑ ☐ ☐
☒ ☑
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: For the first list, Be (Beryllium) has an atomic radius of 111 pm, Be+ (Beryllium ion) has an ionic radius of 31 pm, and Li (Lithium) has an atomic radius of 157 pm.
Since Be+ is a cation, it has a smaller size than both Be and Li. Therefore, the list in order of decreasing size is Li, Be, Be+.
Step 2: For the second list, Br (Bromine) has an atomic radius of 196 pm, I (Iodine) has an atomic radius of 220 pm, and Cl (Chlorine) has an atomic radius of 181 pm.
Therefore, the list in order of decreasing size is I, Br, Cl.
Final Answer
| atoms or ions | atoms or ions in order of decreasing size | | --- | --- | | Be, Be⁺, Li | Li, Be, Be⁺ | | Br, I, Cl | I, Br, Cl | | Cl, Na, S | Cl, Na, S |
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students