QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
What is the chemical formula for the compound strontium sulfide, which contains $S r^{2 +}$ and $S^{2 -}$ ions?
Attachments
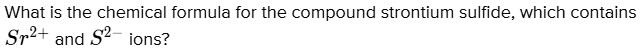
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Determine the proper ratio of ions in the compound.
n \times (+2) = n \times (-2)
The charge of the strontium ion is + 2, and the charge of the sulfide ion is - 2. In order for the compound to be neutral, the total charge of the cations (positive ions) must equal the total charge of the anions (negative ions). Let n be the number of strontium ions; then, the number of sulfide ions is also n, since both have a charge of 2. The product of the charge and the number of ions must be equal for both strontium and sulfide ions:
Step 2: Solve for n.
SrS
Since both sides of the equation are equal, n cancels out: This equation is not solvable, which indicates that the assumption of having only one strontium ion is incorrect. In fact, there must be two strontium ions to balance the charge with two sulfide ions. The proper formula for strontium sulfide is:
Final Answer
The chemical formula for strontium sulfide is SrS.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students