QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
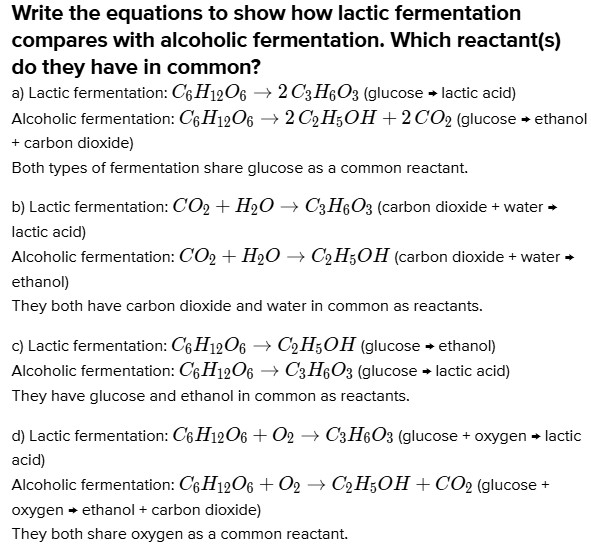
# Write the equations to show how lactic fermentation compares with alcoholic fermentation. Which reactant(s) do they have in common?
a) Lactic fermentation: $C_{6} H_{12} O_{6} \rightarrow 2 C_{3} H_{6} O_{3}$ (glucose $\leftarrow$ lactic acid)
Alcoholic fermentation: $C_{6} H_{12} O_{6} \rightarrow 2 C_{2} H_{5} O H+ 2 C O_{2}$ (glucose $\leftarrow$ ethanol + carbon dioxide)
Both types of fermentation share glucose as a common reactant.
b) Lactic fermentation: $C O_{2}+H_{2} O \rightarrow C_{3} H_{6} O_{3}$ (carbon dioxide + water $\leftarrow$ lactic acid)
Alcoholic fermentation: $C O_{2}+H_{2} O \rightarrow C_{2} H_{5} O H$ (carbon dioxide + water $\leftarrow$ ethanol)
They both have carbon dioxide and water in common as reactants.
c) Lactic fermentation: $C_{6} H_{12} O_{6} \rightarrow C_{2} H_{5} O H$ (glucose $\leftarrow$ ethanol)
Alcoholic fermentation: $C_{6} H_{12} O_{6} \rightarrow C_{3} H_{6} O_{3}$ (glucose $\leftarrow$ lactic acid)
They have glucose and ethanol in common as reactants.
d) Lactic fermentation: $C_{6} H_{12} O_{6}+O_{2} \rightarrow C_{3} H_{6} O_{3}$ (glucose + oxygen $\leftarrow$ lactic acid)
Alcoholic fermentation: $C_{6} H_{12} O_{6}+O_{2} \rightarrow C_{2} H_{5} O H+C O_{2}$ (glucose + oxygen $\leftarrow$ ethanol + carbon dioxide)
They both share oxygen as a common reactant.
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I'll solve this step-by-step using the specified LaTeX formatting guidelines:
Step 2: Analyze the Fermentation Equations
Alcoholic Fermentation: $$C_{6}H_{12}O_{6} \rightarrow 2C_{2}H_{5}OH + 2CO_{2}
Let's carefully examine the equations for lactic and alcoholic fermentation:
Final Answer
Glucose (C_{6}H_{12}O_{6}) is the common reactant in both lactic and alcoholic fermentation.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students