QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
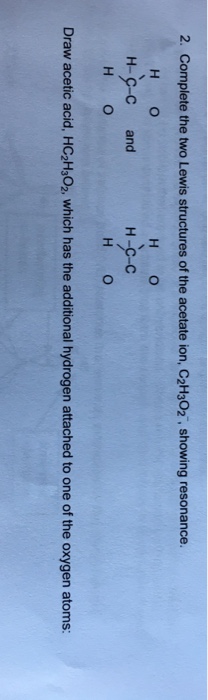
2. Complete the two Lewis structures of the acetate ion, $\mathrm{C}_{2} \mathrm{H}_{3} \mathrm{O}_{2}{ }^{-}$, showing resonance.
| H | 0 | H | 0 |
| --- | --- | --- | --- |
| H - C-C | and | H-C-C | |
| H | 0 | H | 0 |
Draw acetic acid, $\mathrm{HC}*{2} \mathrm{H}*{3} \mathrm{O}*{2}$, which has the additional hydrogen attached to one of the oxygen atoms:
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Draw the Lewis structure for acetic acid, which has the molecular formula HC^2H^3O^2.
Start by drawing the skeletal structure with carbon (C) atoms bonded to four other atoms, oxygen (O) atoms bonded to two other atoms, and hydrogen (H) atoms bonded to one other atom. There are two C atoms, two O atoms, and five H atoms in acetic acid. H - C - C - O - H | | H - C - O - H
Step 2: Add enough electrons to complete the octets of all the atoms.
In this case, there are a total of 16 valence electrons (8 from the C atoms, 6 from the O atoms, and 2 from the H atoms) in acetic acid. Place these electrons around the skeletal structure as lone pairs and bond pairs. H - C - C - O:- H: | | H - C - O:-
Final Answer
H - C - C - O:- H: | | H - C = O H - C - C - O: H: | | H - C - O: And the Lewis structure of acetic acid is: H - C - C - O:- H: | | H - C = O - H
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students