QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
# 2 Question
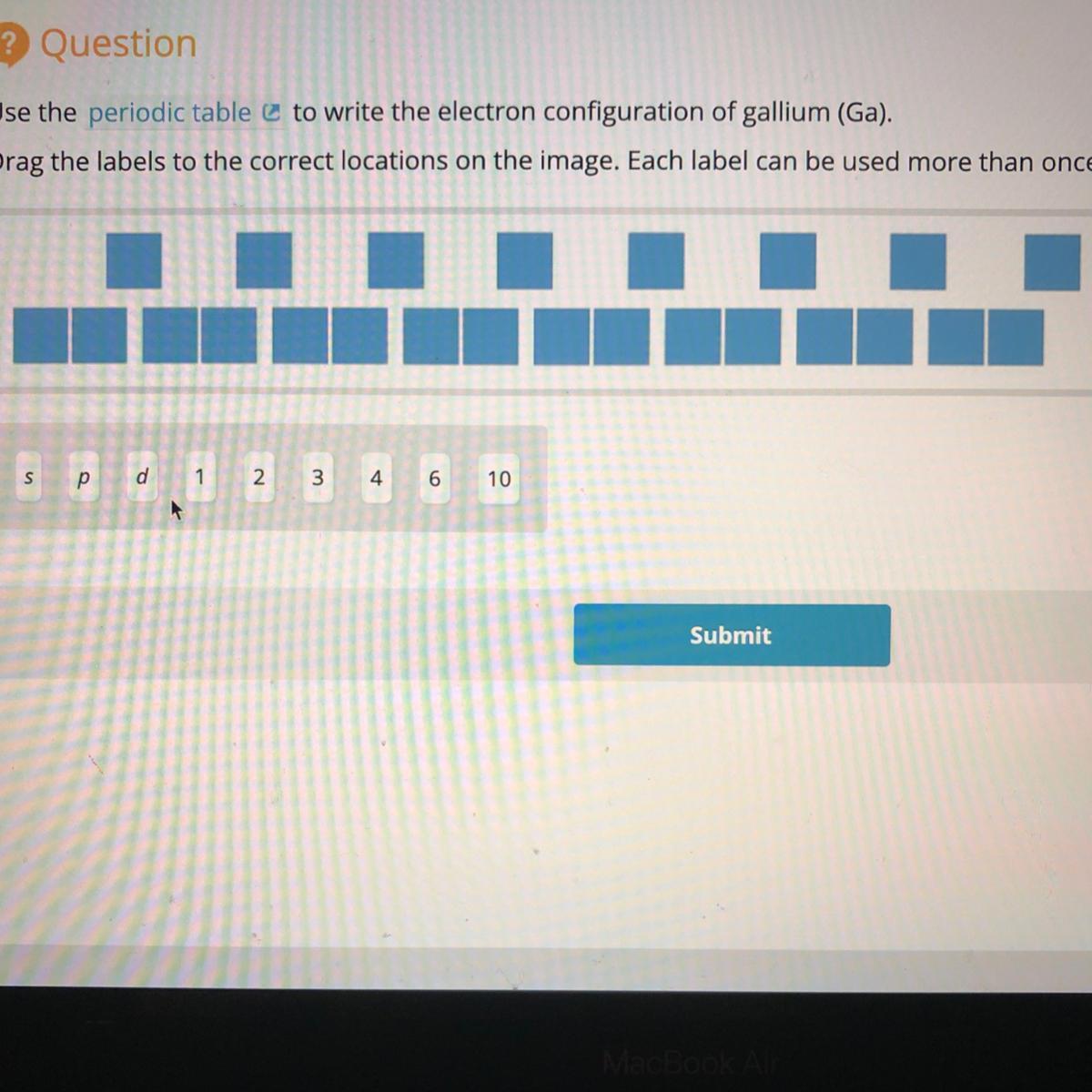
Use the periodic table $\varnothing$ to write the electron configuration of gallium (Ga).
Drag the labels to the correct locations on the image. Each label can be used more than once.
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: First, let's find the atomic number of gallium (Ga) on the periodic table.
The atomic number of Ga is 31.
Step 2: The electron configuration of an element can be determined by filling up the atomic orbitals according to the Aufbau principle, Pauli's exclusion principle, and Hund's rule.
Final Answer
The electron configuration of gallium (Ga) is 1s^{2} \ 2s^{2} \ 2p^{6} \ 3s^{2} \ 3p^{6} \ 4s^{2} \ 3d^{10}.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students