QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
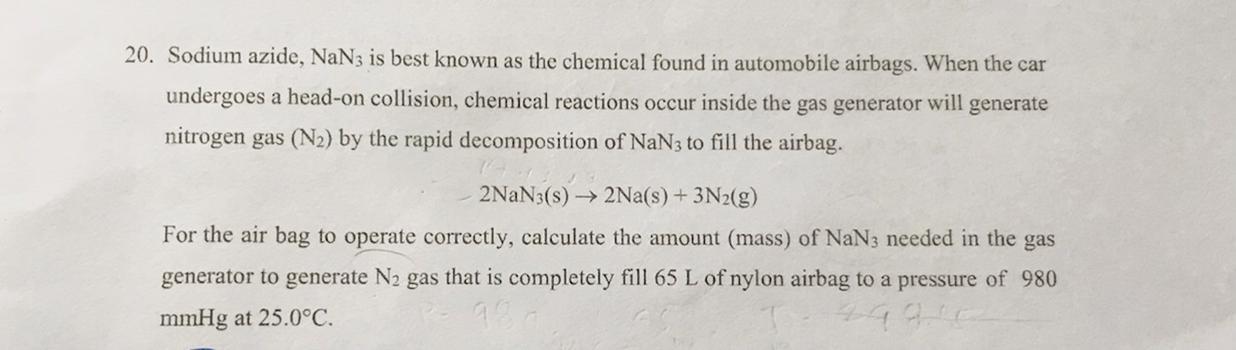
20. Sodium azide, $\mathrm{NaN}_{3}$ is best known as the chemical found in automobile airbags. When the car undergoes a head-on collision, chemical reactions occur inside the gas generator will generate nitrogen gas $\left(\mathrm{N}_{2}\right)$ by the rapid decomposition of $\mathrm{NaN}_{3}$ to fill the airbag.
2 \mathrm{NaN}_{3}(\mathrm{~s}) \rightarrow 2 \mathrm{Na}(\mathrm{~s})+ 3 \mathrm{~N}_{2}(\mathrm{~g})
For the air bag to operate correctly, calculate the amount (mass) of $\mathrm{NaN}_{3}$ needed in the gas generator to generate $\mathrm{N}_{2}$ gas that is completely fill 65 L of nylon airbag to a pressure of 980 mmHg at $25.0^{\circ} \mathrm{C}$.
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Convert the pressure from mmHg to atm.
We know that: 1 atm = 760 mmHg So, 980 mmHg = 980 / 760 = 1.2895 atm (approximately)
Step 2: Use the ideal gas law to calculate the number of moles of N^2 gas.
The ideal gas law is given by: PV = nRT Where: - P is the pressure - V is the volume - n is the number of moles - R is the gas constant - T is the temperature in Kelvin First, convert the temperature to Kelvin: T = 25.0^{\circ} \mathrm{C} + 273.15 = 298.15 \mathrm{~K} Now, we can calculate the number of moles of N^2 gas:
Final Answer
The amount of NaN^3 required is 14.495 g (approximately).
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students