QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
| 4 | Name the following compound: $\mathrm{N}_{2} \mathrm{O}_{5}$. <br> nitrogen oxide <br> Dinitrogenpentoxide <br> Dinitrogen hexoxide <br> Dinitrogen octaoxide <br> Dinitrogen fiveoxide |
| :--: | :--: |
Attachments
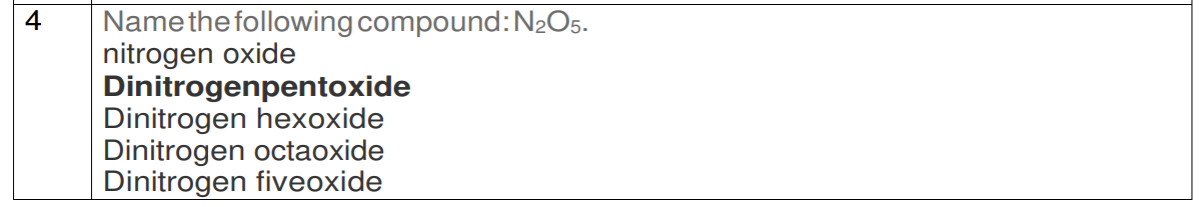
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the prefixes in the compound name to find the number of atoms in each element.
The compound's name is Dinitrogenpentoxide. The prefix "di-" means there are 2 atoms of nitrogen (N), and the prefix "penta-" means there are 5 atoms of oxygen (O).
Step 2: Write the chemical formula based on the number of atoms for each element.
Since there are 2 nitrogen atoms and 5 oxygen atoms, the chemical formula is $\mathrm{N}_{2} \mathrm{O}_{5}$.
Final Answer
The compound's name is Dinitrogenpentoxide, and its chemical formula is $\mathrm{N}_{2} \mathrm{O}_{5}$.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students