QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
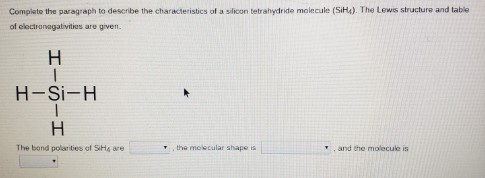
Complete the paragraph to describe the characteristics of a silicon tetrahydride molecule (SiH4). The Lewis structure and table of electronegativities are given.
\begin{array}{cccc}
\text{H} & & \\
& \text{H} \text{Si} \text{-H} & & \\
& \text{H} & & \\
\text { The bond polarities of } \mathrm{SiH}_{2} \text { are } & \bullet \text {, the molecular shape is } & \bullet \text {, and the molecule is }
\end{array}
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the Lewis structure and electronegativity values.
The Lewis structure for silicon tetrahydride (SiH4) is given in the problem. It shows that silicon (Si) is in the center, surrounded by four hydrogen (H) atoms, with each bond being a single covalent bond. From the table of electronegativities, we find that the electronegativity value for silicon (Si) is approximately 1.8 and for hydrogen (H) is approximately 2.2.
Step 2: Analyze bond polarity.
Bond polarity depends on the difference in electronegativity between the two atoms forming the bond. In this case, the bond is between silicon and hydrogen (Si-H). Since the difference in electronegativity (2.2 - 1.8 = 0.4) is less than 0.5, the bond is considered nonpolar.
Final Answer
The bond polarities of SiH^4 are nonpolar (Si-H), the molecular shape is tetrahedral, and the molecule is nonpolar.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students