QQuestionChemistry
QuestionChemistry
Determine the number of protons, electrons, and neutrons present in an atom of calcium (Ca). Explain how you determined your answer using complete sentences.
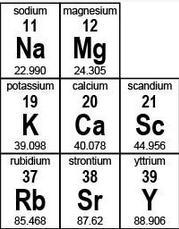
| **sodium 11 Na 22.990 ** | **magnesium 12 Mg 24.305 ** | |
| --- | --- | --- |
| potassium 19 K 39.098 | calcium 20 Ca 40.078 | scandium 21 Sc 44.956 |
| rubidium 37 Rb 85.468 | strontium 38 Sr 87.62 | yttrium 39 Y 88.906 |
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: To determine the number of protons, electrons, and neutrons in an atom of calcium (Ca), we first need to find out its atomic number and atomic mass.
Step 2: The atomic number of calcium is 20, which can be found in the periodic table provided.
\text{Atomic number of Ca} = 20 \Rightarrow \text{Number of protons in Ca} = 20
The atomic number represents the number of protons in an atom.
Final Answer
- Number of protons in Ca: 20 - Number of electrons in Ca: 20 - Number of neutrons in Ca: 20
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students