QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
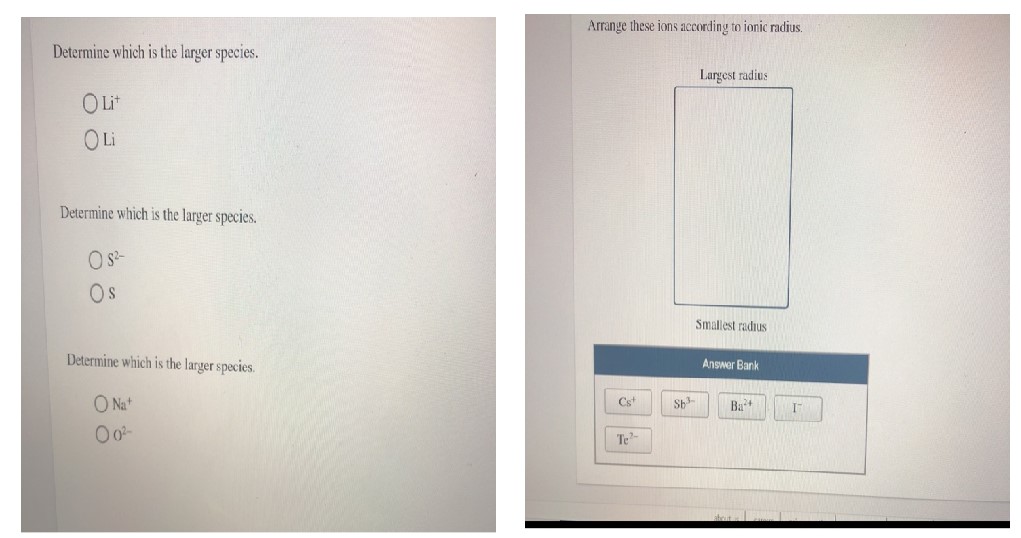
Determine which is the larger species.
$\mathrm{Li}^{+}$
Li
Determine which is the larger species.
$\mathrm{S}^{2 -}$
S
Determine which is the larger species.
$\mathrm{Na}^{+}$
$\mathrm{O}^{2 -}$
Arrange these ions according to ionic radius.
Largest radius
Smallest radius
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Understand the problem
We are given three pairs of ions and asked to determine which ion in each pair has the larger radius. We also need to arrange the given ions according to their increasing ionic radii.
Step 2: Recall the trend of ionic radii in the periodic table
Ionic radius generally decreases when going from left to right along a period (row) in the periodic table and increases when going down a group (column).
Final Answer
- The larger species in the first pair is Li. - The larger species in the second pair is S^(2 -). - The larger species in the third pair is O^(2 -). - The given ions arranged according to increasing ionic radius are: Li+, Li, Na+, S, O^(2 -).
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students