QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
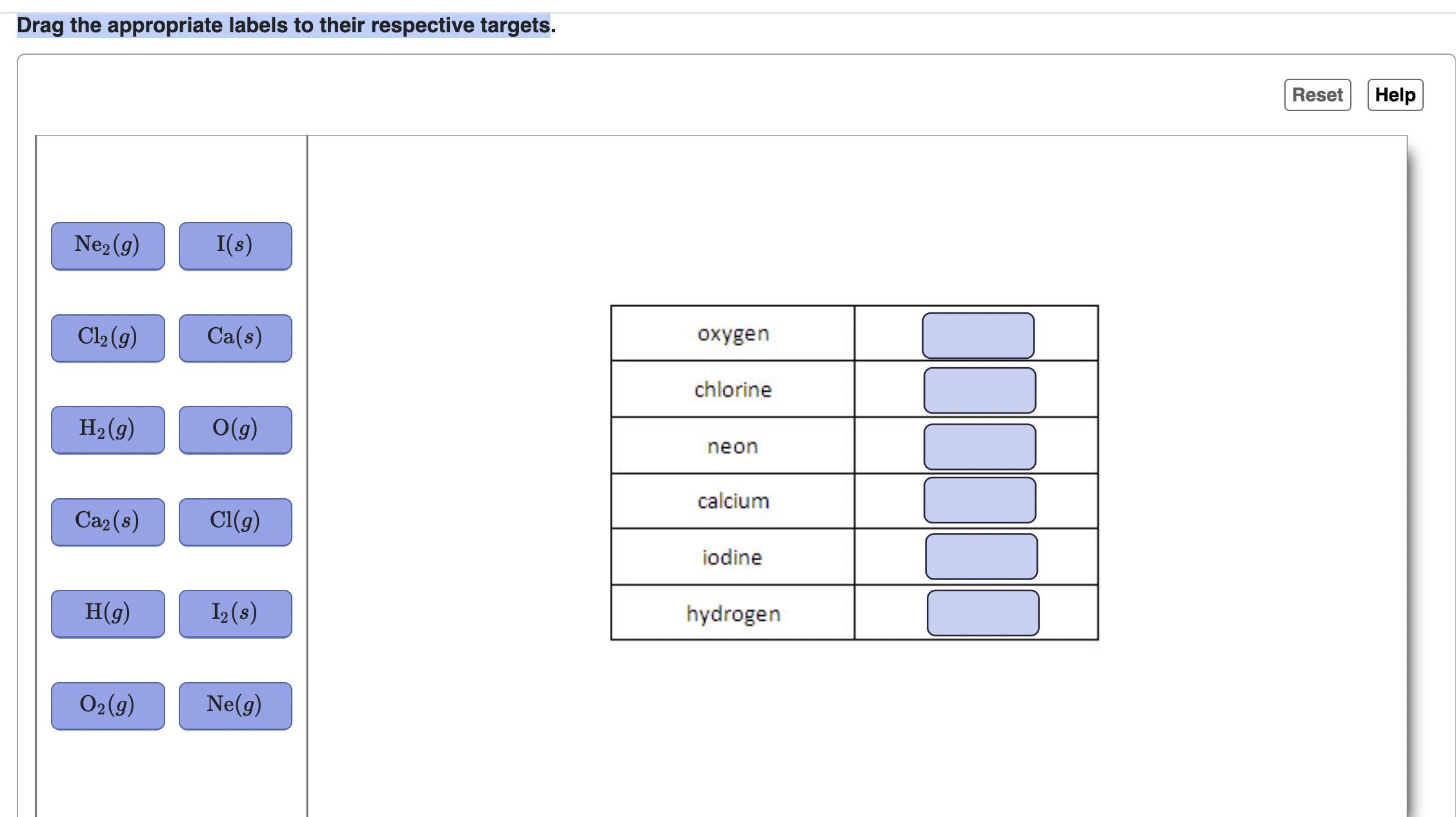
# Drag the appropriate labels to their respective targets.
| Reset | Help |
| --- | --- |
| | |
| Ne₂(g) | I(s) |
| --- | --- |
| | |
| Cl₂(g) | Ca(s) |
| --- | --- |
| | |
| H₂(g) | O(g) |
| --- | --- |
| | |
| Ca₂(s) | Cl(g) |
| --- | --- |
| | |
| H(g) | I₂(s) |
| --- | --- |
| | |
| O₂(g) | Ne(g) |
| --- | --- |
| | |
| oxygen | |
| --- | --- |
| chlorine | |
| neon | |
| calcium | |
| iodine | |
| hydrogen | |
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I will solve the chemical equation puzzle by balancing the chemical equation for each target box.
Step 2: Identify the given substances and products in each box.
For the first box, the given substances are Ne₂(g) and I(s), and the product is I₂(s). For the second box, the given substances are Cl₂(g) and Ca(s), and the product is CaCl₂(s). For the third box, the given substances are H₂(g) and O(g), and the product is H₂O(g).
Final Answer
The balanced chemical equations for each box are: 1. \text{Ne}_{2(g)} + 2 \text{I}_{(s)} \rightarrow 2 \text{I}_{2(s)} 2. \text{Cl}_{2(g)} + \text{Ca}_{(s)} \rightarrow \text{CaCl}_{2(s)} 3. \text{H}_{2(g)} + \frac{1}{2} \text{O}_{2(g)} \rightarrow \text{H}_2\text{O}_{(g)} 4. \text{Ca}_{2(s)} + 2 \text{Cl}_{(g)} \rightarrow 2 \text{CaCl}_{2(s)} 5. \text{H}_{(g)} + \text{I}_{2(s)} \rightarrow 2 \text{HI}_{(g)} 6. No reaction.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students