QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
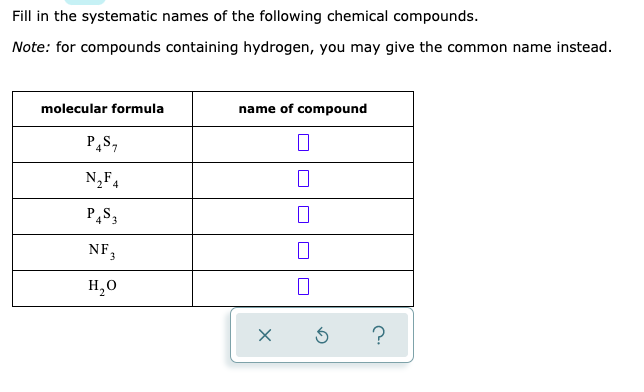
Fill in the systematic names of the following chemical compounds. Note: for compounds containing hydrogen, you may give the common name instead.
| molecular formula | name of compound |
| --- | --- |
| $\mathrm{P}*{4} \mathrm{~S}*{7}$ | $\square$ |
| $\mathrm{N}*{2} \mathrm{~F}*{4}$ | $\square$ |
| $\mathrm{P}*{4} \mathrm{~S}*{3}$ | $\square$ |
| $\mathrm{NF}_{3}$ | $\square$ |
| $\mathrm{H}_{2} \mathrm{O}$ | $\square$ |
\times 5 ?
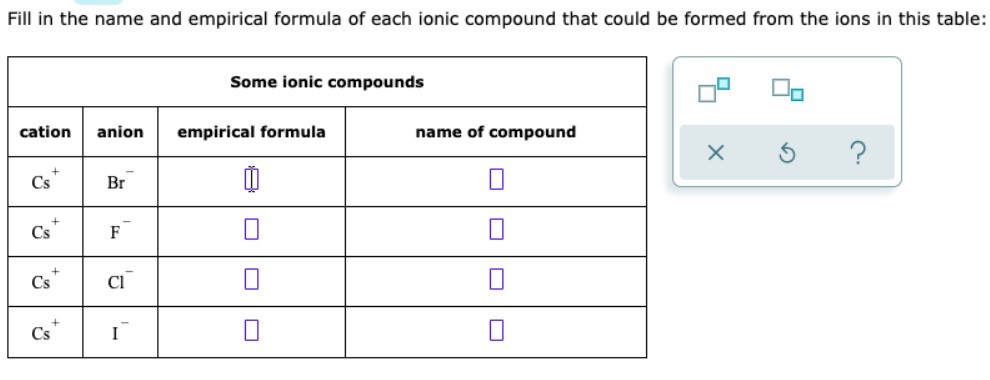
Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table:
| Some ionic compounds | | | |
| --- | --- | --- | --- |
| cation | anion | empirical formula | name of compound |
| $\mathrm{Cs}^{+}$ | $\mathrm{Br}^{-}$ | 1 | $\square$ |
| $\mathrm{Cs}^{+}$ | $\mathrm{F}^{-}$ | $\square$ | $\square$ |
| $\mathrm{Cs}^{+}$ | $\mathrm{Cl}^{-}$ | $\square$ | $\square$ |
| $\mathrm{Cs}^{+}$ | $\mathrm{I}^{-}$ | $\square$ | $\square$ |
| $\square^{\square}$ | $\square^{\square}$ | | | | | $\times$ | $5$ | $?$ | | :-- | :-- | :-- | :-- | :-- | :-- | :-- | :-- | :-- | :-- | :-- | :-- | :-- |
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Fill in the systematic names of the following chemical compounds.
| $\mathrm{H}_2 \mathrm{O}$ | water |
Note: for compounds containing hydrogen, you may give the common name instead. | molecular formula | name of compound | | --- | --- |
Step 2: Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table:
| $\mathrm{Cs}^{+}$ | $\mathrm{I}^{-}$ | $\mathrm{CsI}$ | cesium iodide |
| cation | anion | empirical formula | name of compound | | --- | --- | --- | --- |
Final Answer
1. Tetraphosphorus heptasulfide, dinitrogen tetrafluoride, tetraphosphorus trisulfide, nitrogen trifluoride, water 2. Cesium bromide, cesium fluoride, cesium chloride, cesium iodide
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students