QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
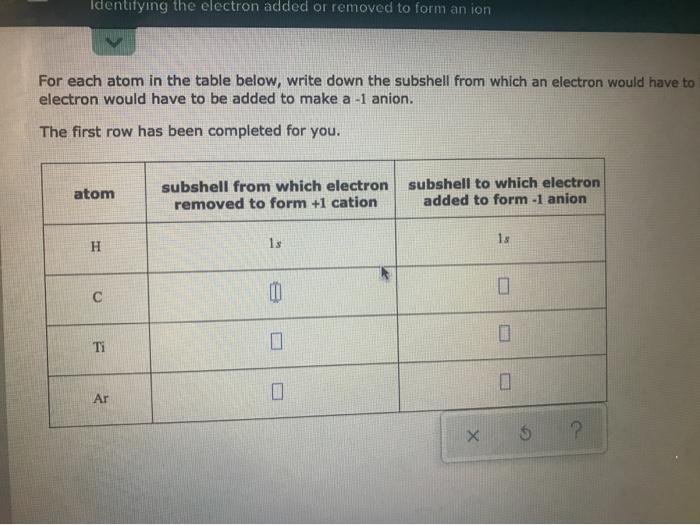
For each atom in the table below, write down the subshell from which an electron would have to electron would have to be added to make a - 1 anion.
The first row has been completed for you.
| atom | subshell from which electron removed to form + 1 cation | subshell to which electron added to form - 1 anion |
| --- | --- | --- |
| H | 1x | 1x |
| C | | |
| Ti | | |
| Ar | | |
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: To determine the subshell to which an electron must be added to form a - 1 anion, we need to find the outermost subshell in each atom.
This is the subshell that can accommodate one more electron to complete the octet and form a stable - 1 anion.
Step 2: For Carbon (C), the atomic number is 6, and its electron configuration is 1s^2 2s^2 2p^2.
The outermost subshell is $$2p$$.
Therefore, an electron must be added to the 2p subshell to form a - 1 anion.
Final Answer
| atom | subshell from which electron removed to form + 1 cation | subshell to which electron added to form - 1 anion | | --- | --- | --- | | H | 1s | 1s | | C | 2s or 2p | 2p | | Ti | 4s or 3d or 4p | 4d | | Ar | 3p | 3p | Note: For Carbon, either the 2s or 2p subshell can be used to form a + 1 cation, depending on the specific chemical reaction. Similarly, for Titanium, either the 4s, 3d, or 4p subshell can be used to form a + 1 cation, depending on the specific chemical reaction.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students