QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
How many valence electrons does the element with the following electron configuration have?
1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4 s^{2} 3 d^{10} 4 p^{4}
Attachments
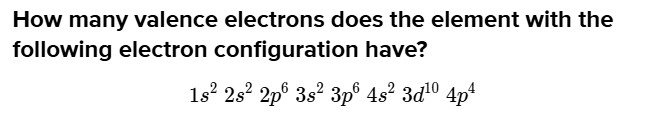
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1Let's solve this step by step:
Step 2: Understand Valence Electrons
Valence electrons are the electrons in the outermost shell of an atom, which participate in chemical bonding. These are typically the electrons in the highest energy level and subshells.
Final Answer
The element has 6 valence electrons.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students