QQuestionChemistry
QuestionChemistry
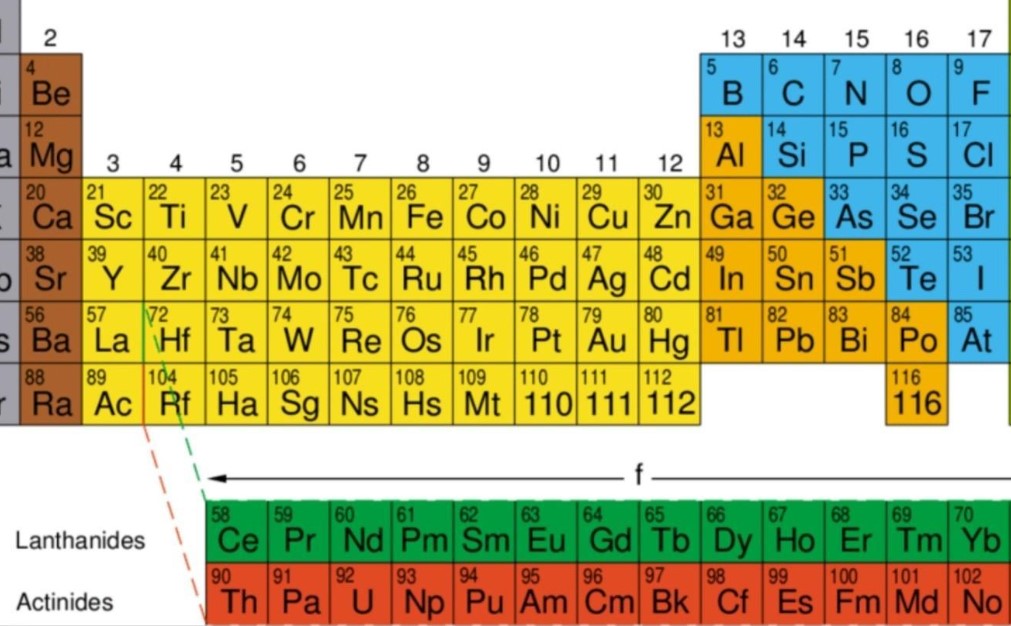
In the modern periodic table, find the element named lead (Pb). How many protons do atoms of lead have? To which class of elements does lead belong?
| 1 | 2 | | | | | | | | | | | | | | | 13 | 14 | 15 | 16 | 17 |
| --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 2 | | | | | | | | | | | | | | | | | | 5 | 6 | 7 |
| 3 | Be | | | | | | | | | | | | | | | | | B | C | N |
| 4 | 12 | | | | | | | | | | | | | | | | | 13 | 14 | 15 |
| 5 | Mg | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | | | | | | | Al | Si | P |
| 6 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | | | | | | | 31 | 32 | 33 |
| 7 | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | | | | | | | Ga | Ge | As |
| 8 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | | | | | | | 49 | 50 | 51 |
| 9 | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | | | | | | | In | Sn | Sb |
| 10 | 56 | 57 | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 | | | | | | | 81 | 82 | 83 |
| 11 | Ba | La | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | | | | | | | Tl | Pb | Bi |
| 12 | 88 | 89 | 104 | 105 | 106 | 107 | 108 | 109 | 110 | 111 | 112 | | | | | | | | 116 | |
| 13 | Ra | Ac | Rf | Ha | Sg | Ns | Hs | Mt | 110 | 111 | 112 | | | | | | | | 116 | |
Lanthanides
Actinides
f
| 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 |
| --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb |
| 90 | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | 100 | 101 | 102 |
| Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No |
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I'll solve this problem step by step using the specified LaTeX formatting guidelines:
Step 2: Locate Lead (Pb) on the Periodic Table
- From the periodic table, I can find lead (Pb) in row 11, column 18 - The atomic number (number of protons) is located directly below the element
Final Answer
- Number of Protons: 82 - Element Class: Post-transition metal (p-block element)
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students