QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
| # of C atoms | Stem | # of C atoms | Stem |
| --- | --- | --- | --- |
| 1 | Meth | 11 | undec |
| 2 | Eth | 12 | dodec |
| 3 | Prop | 13 | Tridec |
| 4 | But | 14 | Tetradec |
| 5 | Pent | 15 | Pentadec |
| 6 | Hex | 16 | Hexadec |
| 7 | Hept | 17 | Heptadec |
| 8 | Oct | 18 | Octadec |
| 9 | Non | 19 | Nonadec |
| 10 | Dec | 20 | Eicos |
The isomers must be named as a substituted decane and will be called ***_***____.
The names of C₆H₁₂ and C₆H₁₄ isomers that contain one carbon bounded only to other carbons contain the structural prefix "neo". Like "iso" is not separated from the alkane portion of the name.
The IUPAC name for ***_***____ is ***_***____.
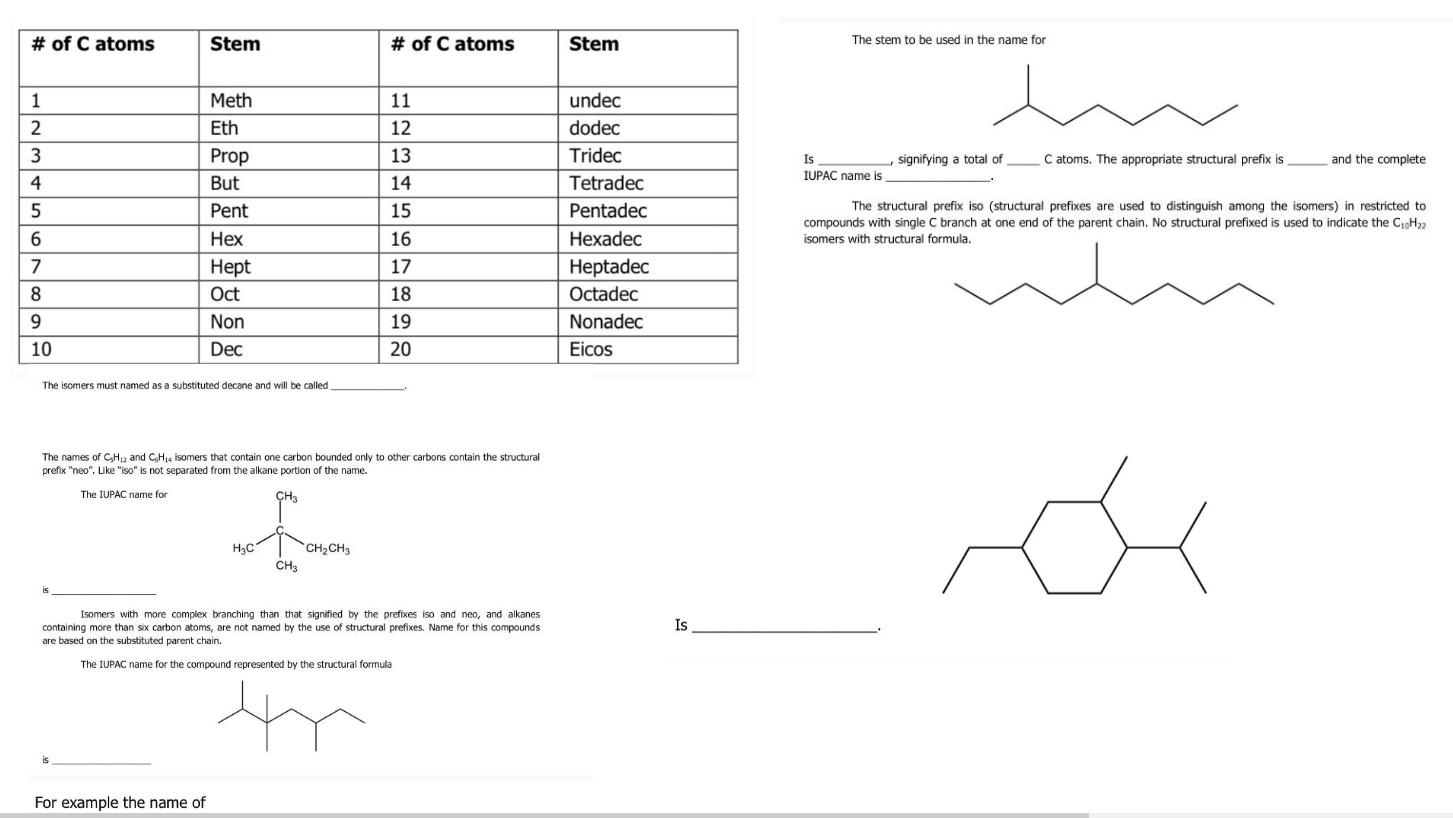
Is ***_***____, signifying a total of ***_***____. The appropriate structural prefix is ***_***____ and the complete IUPAC name is ***_***____.
The structural prefix is (structural prefixes are used to distinguish among the isomers) is restricted to compounds with single C branch at one end of the parent chain. No structural prefix is used to indicate the C₁₀H₁₀ isomers with structural formula.
For example the name of ***_***____ is ***_***____.
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I will solve the given homework problem step by step, following the formatting guidelines exactly.
The problem involves naming certain organic compounds, specifically decane isomers with a substituted carbon chain.
Step 2: Identify the number of carbon atoms in the substituted chain.
The number of carbon atoms in the substituted chain is provided in the table. For this example, let's consider the isomer with 7 carbon atoms in the substituted chain, which corresponds to heptadecane (C^17H^35).
Final Answer
The IUPAC name for the given decane isomer is "4 -heptadecyldecane".
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students