QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
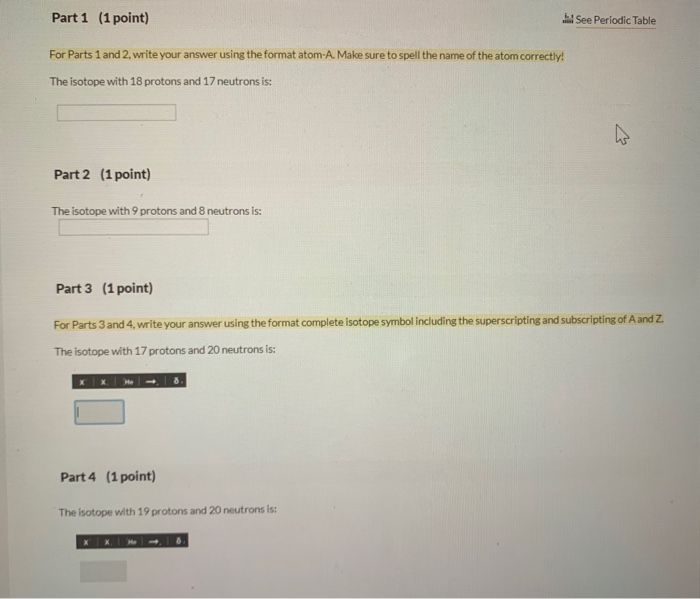
# Part 1 (1 point)
For Parts 1 and 2, write your answer using the format `atom-A`. Make sure to spell the name of the atom correctly!
The isotope with 18 protons and 17 neutrons is:
# Part 2 (1 point)
The isotope with 9 protons and 8 neutrons is:
# Part 3 (1 point)
For Parts 3 and 4, write your answer using the format `complete isotope symbol` including the superscripting and subscripting of A and Z.
The isotope with 17 protons and 20 neutrons is:
# Part 4 (1 point)
The isotope with 19 protons and 20 neutrons is:
# Part 5 (1 point)
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the number of protons and neutrons for each isotope.
Part 1: The isotope with 18 protons and 17 neutrons is Oxygen- 18. The number of protons (also known as atomic number) determines the element, while the sum of protons and neutrons (also known as mass number) determines the isotope. Part 2: The isotope with 9 protons and 8 neutrons is Fluorine- 17.
Step 2: Write the isotope symbol using the correct format.
Part 3: The isotope with 17 protons and 20 neutrons is Argon- 37. The atomic number Z (number of protons) is 17, and the mass number A (sum of protons and neutrons) is 37. _{17}^{37}\text{Ar} Part 4: The isotope with 19 protons and 20 neutrons is Potassium- 39. The atomic number Z (number of protons) is 19, and the mass number A (sum of protons and neutrons) is 39. _{19}^{39}\text{K}
Final Answer
Part 1: Oxygen- 18 Part 2: Fluorine- 17 Part 3: Argon- 37 Part 4: Potassium- 39
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students