QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
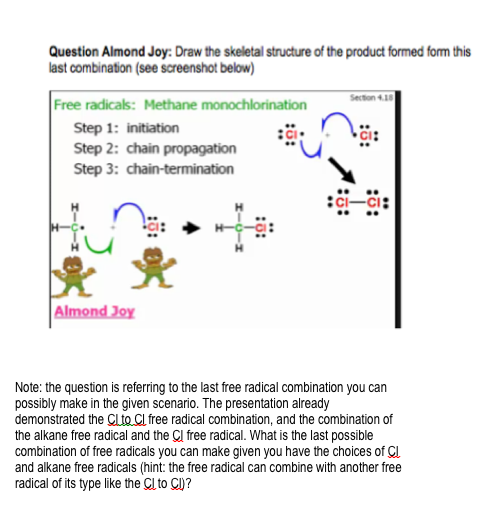
Question Almond Joy: Draw the skeletal structure of the product formed form this last combination (see screenshot below)
Note: the question is referring to the last free radical combination you can possibly make in the given scenario. The presentation already demonstrated the Cl to Cl free radical combination, and the combination of the alkane free radical and the Cl free radical. What is the last possible combination of free radicals you can make given you have the choices of Cl and alkane free radicals (hint: the free radical can combine with another free radical of its type like the Cl to Cl)?
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the remaining free radicals that can combine.
In this case, we have two types of free radicals remaining: alkane free radicals and chlorine (Cl) free radicals.
Step 2: Understand the free radical combination process.
A free radical is a highly reactive species with an unpaired electron. When two free radicals combine, they share their unpaired electrons to form a stable covalent bond.
Final Answer
The skeletal structure of the product formed from the last free radical combination is CH^3 -CH^3, representing the formation of an ethane molecule (C^2H6) through the combination of two methyl free radicals.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students