QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
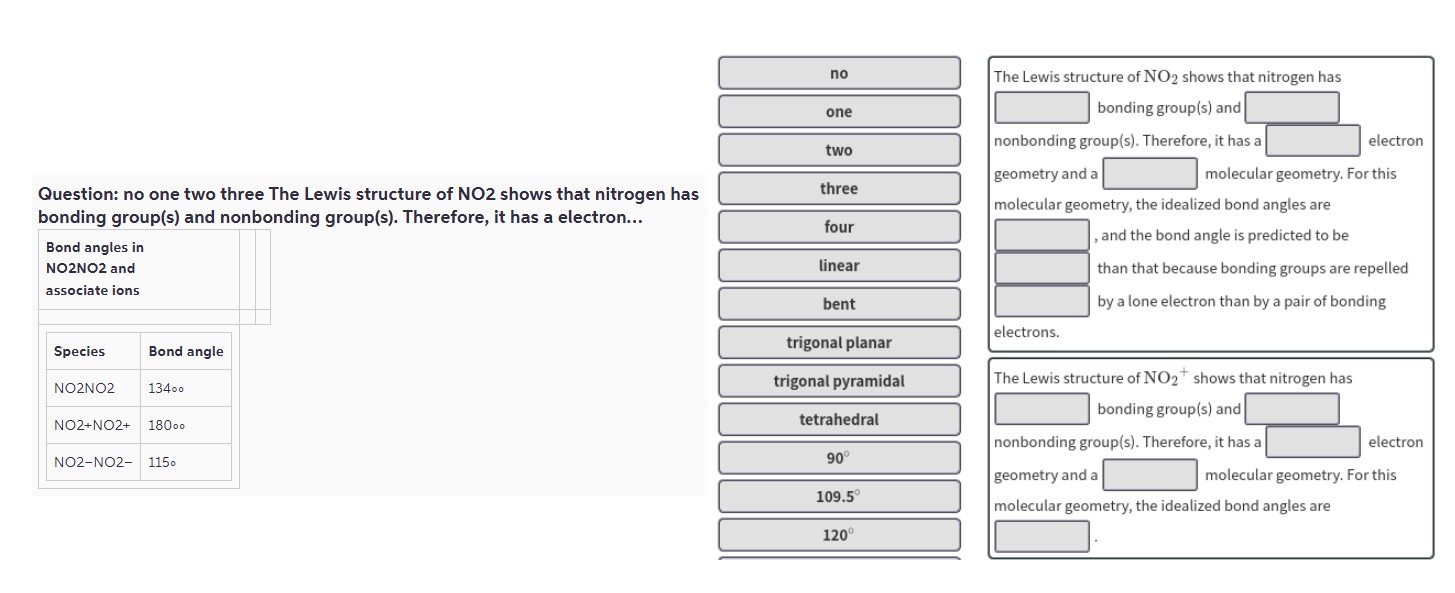
Question: no one two three The Lewis structure of NO^2 shows that nitrogen has bonding group(2$) and nonbonding group(2$). Therefore, it has a electron...
| Bond angles in NO^2NO^2 and associate ions | |
| --- | --- |
| Species | Bond angle |
| NO^2NO^2 | 134∘ |
| NO^2 +NO^2 + | 180∘ |
| NO^2 -NO^2 - | 115∘ |
| no | |
| --- | --- |
| one | |
| two | |
| three | |
| four | |
| linear | |
| bent | |
| trigonal planar | |
| trigonal pyramidal | |
| tetrahedral | |
| 90° | |
| 109.5° | |
| 120° | |
The Lewis structure of NO^2 shows that nitrogen has bonding group(2$) and nonbonding group(2$). Therefore, it has a electron geometry and a molecular geometry. For this molecular geometry, the idealized bond angles are , and the bond angle is predicted to be than that because bonding groups are repelled by a lone electron than by a pair of bonding electrons.
The Lewis structure of NO^2 + shows that nitrogen has bonding group(2$) and nonbonding group(2$). Therefore, it has a electron geometry and a molecular geometry. For this molecular geometry, the idealized bond angles are .
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the molecular geometry of NO^2 and NO^2 +.
The molecular geometry of NO^2 is bent, and the molecular geometry of NO^2 + is linear.
Step 2: Identify the ideal bond angles for each molecular geometry.
For a bent molecular geometry, the ideal bond angle is 120°. For a linear molecular geometry, the ideal bond angle is 180°.
Final Answer
The molecular geometry of NO^2 is bent, with an ideal bond angle of 120° and a measured bond angle of 134°. The molecular geometry of NO^2 + is linear, with an ideal bond angle of 180° and a measured bond angle of 180°. The difference in bond angles is due to the repulsion between bonding groups and lone electron pairs.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students