QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
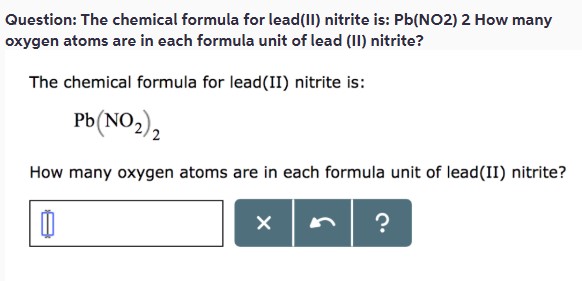
**Question:** The chemical formula for lead(II) nitrite is: Pb(NO₂)₂. How many oxygen atoms are in each formula unit of lead(II) nitrite?
The chemical formula for lead(II) nitrite is:
Pb(NO_2)_2
How many oxygen atoms are in each formula unit of lead(II) nitrite?
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1**Step 1:** Identify the number of oxygen atoms in the nitrite ion, NO^2.-
The nitrite ion has one nitrogen atom and two oxygen atoms. This is represented by the subscript 2 in the formula NO^2 -. **Step 2:** Examine the subscript of the nitrite ion in the overall formula for lead(2$) nitrite. In the formula for lead(2$) nitrite, there are two nitrite ions present, as indicated by the subscript 2 after NO^2 -. **Step 3:** Multiply the number of oxygen atoms in the nitrite ion by the number of nitrite ions in the formula. Since there are 2 oxygen atoms in each nitrite ion, and there are 2 nitrite ions in the formula for lead(2$) nitrite, there are a total of 2 x 2 = 4 oxygen atoms in each formula unit of lead(2$) nitrite. **
Final Answer
** There are 4 oxygen atoms in each formula unit of lead(II) nitrite.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students