QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
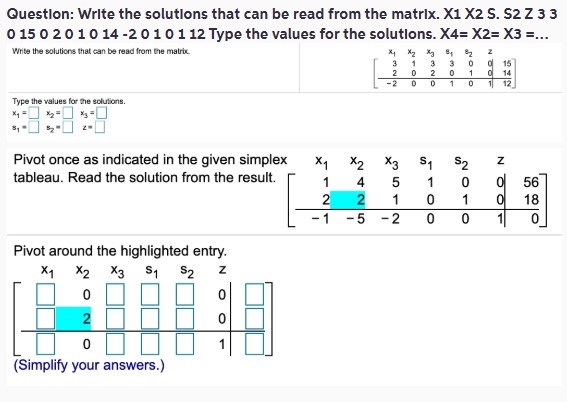
**Question: Write the solutions that can be read from the matrix. X^1 X^2 S. S^2 Z 3 3 0 15 0 2 0 1 0 14 - 2 0 1 0 1 12 Type the values for the solutions. X^4 = X^2 = X^3 = ...**
Write the solutions that can be read from the matrix.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the variables corresponding to each column in the matrix.
\begin{bmatrix}
We can rewrite the matrix with these variables: 1 & 0 & 0 & 0 & 0 & 3 \ 0 & 1 & 0 & 0 & 0 & 3 \ 0 & 0 & 1 & 0 & 0 & 0 \ 0 & 0 & 0 & 1 & 0 & 2 \ 0 & 0 & 0 & 0 & 1 & 14 \ 0 & 0 & 0 & 1 & 0 & 1 \ 0 & 1 & 0 & 0 & 0 & 12 \end{bmatrix}
Step 2: Write the solutions for each variable.
- $Z = 14$
From the matrix, we can see that:
Final Answer
- $X\_1 = 1$ - $X\_2 = 1$ - $S = 1$ - $S\_2 = 1$ - $Z = 1$ - $X\_1$ and $X\_1$ are equal and dependent on the same value.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students