QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
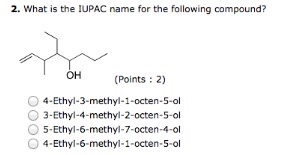
2. What is the IUPAC name for the following compound?
(Points: 2)
- 4 -Ethyl- 3 -methyl- 1 -octen- 5 -ol
- 3 -Ethyl- 4 -methyl- 2 -octen- 5 -ol
- 5 -Ethyl- 6 -methyl- 7 -octen- 4 -ol
- 4 -Ethyl- 6 -methyl- 1 -octen- 5 -ol
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the longest continuous carbon chain in the molecule.
The longest continuous carbon chain in this molecule contains 8 carbon atoms, making it an octane (or octen) chain. We can see that the chain is numbered from left to right, with the double bond on the third carbon atom and the alcohol functional group on the fifth carbon atom.
Step 2: Identify and number the substituents on the carbon chain.
There are two substituents on this carbon chain: an ethyl group (−C^2H5) and a methyl group (−CH3). The ethyl group is attached to the fourth carbon atom of the chain, and the methyl group is attached to the third carbon atom. Therefore, the substituents can be named as follows: - Ethyl group: −C^2H^5 (attached to the 4th carbon atom) - Methyl group: −CH^3 (attached to the 3rd carbon atom)
Final Answer
4 -ethyl- 3 -methyl- 1 -octen- 5 -ol
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students