QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
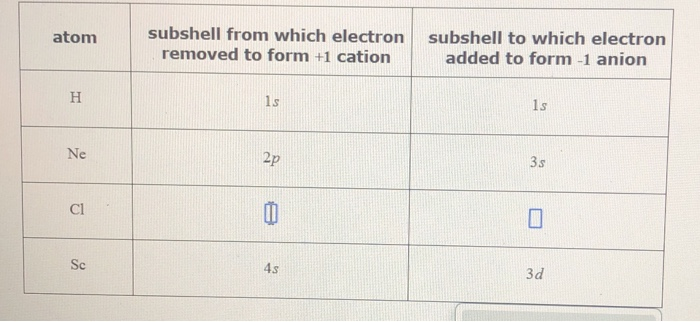
| atom | subshell from which electron removed to form + 1 cation | subshell to which electron added to form - 1 anion |
| --- | --- | --- |
| H | 1s | 1s |
| Ne | 2p | 3s |
| Cl | 11 | 1 |
| Sc | 4s | 3d |
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the subshell from which an electron is removed to form a cation and the subshell to which an electron is added to form an anion for each atom.
The given table provides the information needed. We will analyze each row and find the corresponding subshells.
Step 2: Analyze the first row (Hydrogen atom).
For a Hydrogen atom (H) to form a + 1 cation, an electron is removed from the 1s subshell. To form a - 1 anion, an electron is added to the 1s subshell.
Final Answer
| atom | subshell from which electron removed to form + 1 cation | subshell to which electron added to form - 1 anion | | --- | --- | --- | | H | 1s | 1s | | Ne | 2p | 3s | | Cl | 3p | 1s (does not exist, so 2s, 2p, or 3s instead) | | Sc | 4s | 3d |
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students