QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
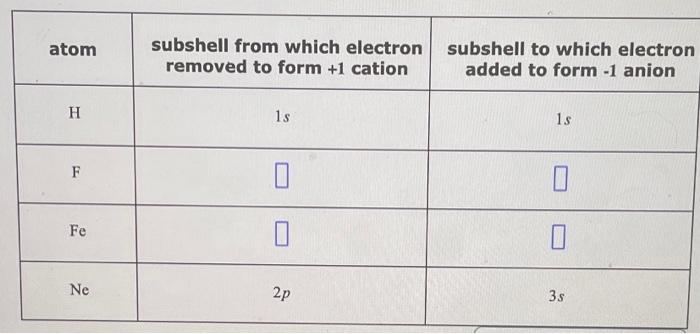
| atom | subshell from which electron removed to form + 1 cation | subshell to which electron added to form - 1 anion |
| --- | --- | --- |
| H | $1 s$ | $1 s$ |
| F | | |
| Fe | | |
| Ne | $2 p$ | $3 s$ |
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the electron configuration for the given atoms.
- Neon (Ne): 1s$^2$ 2s$^2$ 2p$^6$ 3s$^2$ 3p$^6$
Step 2: Determine the subshell from which an electron is removed to form a + 1 cation.
- Neon (Ne$^+$): An electron is removed from the 3s subshell to form a +1 cation: 1s$^2$ 2s$^2$ 2p$^6$ 3s$^1$
Final Answer
- H$^+$: 1s$^1$ - F$^+$: 1s$^1$ 2s$^1$ 2p$^1$ - Fe$^+$: 1s$^1$ 2s$^1$ 2p$^1$ 3s$^1$ 3p$^1$ 3d$^1$ 4s$^1$ - Ne$^+$: 1s$^1$ 2s$^1$ 2p$^1$ 3s$^1$ - H$^-$: 1s$^1$ - F$^-$: 1s$^1$ 2s$^1$ 2p$^1$ - Fe$^-$: 1s$^1$ 2s$^1$ 2p$^1$ 3s$^1$ 3p$^1$ 3d$^1$ 4s$^1$ - Ne$^-$: 1s$^1$ 2s$^1$ 2p$^1$ 3s$^1$
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students