QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
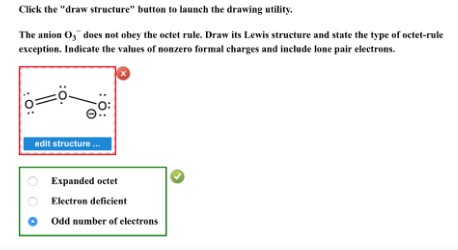
Click the "draw structure" button to launch the drawing utility.
The anion $\mathrm{O}_{2}{ }^{-}$does not obey the octet rule. Draw its Lewis structure and state the type of octet-rule exception. Indicate the values of nonzero formal charges and include lone pair electrons.
# 00000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Determine the total number of valence electrons in the $\mathrm{O}_{2}{ }^{-}$ anion.
The $\mathrm{O}_{2}{ }^{-}$ anion has 2 oxygen atoms, so there are a total of 2 * 6 = 12 valence electrons.
Oxygen (O) is in period 2 and group 16 of the periodic table, so it has 6 valence electrons.
Step 2: Draw a skeletal structure for the molecule.
\mathrm{O}=\mathrm{O}
Place the two oxygen atoms next to each other, sharing a double bond:
Final Answer
\mathrm{O}:\stackrel{.}{e}:\stackrel{.}{e}:\mathrm{O}^{-}
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students