QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
Complete the equation for the reaction of baking soda (sodium bicarbonate, NaHCO3) with vinegar (acetic acid, HC^2H^3O2). Include phase symbols.
\begin{aligned}
& \mathrm{NaHCO}_{3}(s)+\mathrm{HC}_{2} \mathrm{H}_{3} \mathrm{O}_{2}(a q) \rightarrow \mathrm{NaC}_{2} \mathrm{H}_{3} \mathrm{O}_{2}(a q)+\mathrm{H}_{2} \mathrm{O}(l)+\mathrm{CO}_{2}(g) \\
& \text {
Attachments
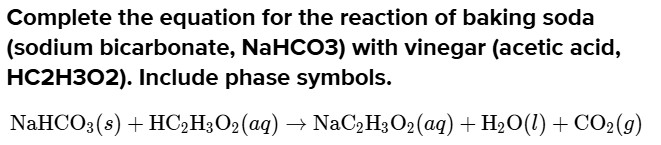
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the reactants and products in the given chemical equation.
The reactants are sodium bicarbonate (NaHCO^3(s)) and acetic acid (HC^2H^3O^2(aq)). The products are sodium acetate (NaC^2H^3O^2(aq)), water (H^2O(l)), and carbon dioxide (CO^2(g))).
Step 2: Write the complete chemical equation with phase symbols.
NaHCO^3(s) + HC^2H^3O^2(aq) -> NaC^2H^3O^2(aq) + H^2O(l) + CO^2(g)
Final Answer
\mathrm{NaHCO}_{3}(s)+\mathrm{HC}_{2} \mathrm{H}_{3} \mathrm{O}_{2}(a q) \rightarrow \mathrm{NaC}_{2} \mathrm{H}_{3} \mathrm{O}_{2}(a q)+\mathrm{H}_{2} \mathrm{O}(l)+\mathrm{CO}_{2}(g)
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students