QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
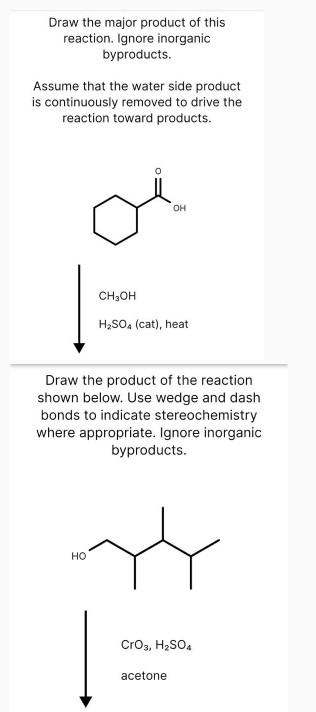
Draw the major product of this reaction. Ignore inorganic byproducts.
Assume that the water side product is continuously removed to drive the reaction toward products.
Draw the product of the reaction shown below. Use wedge and dash bonds to indicate stereochemistry where appropriate. Ignore inorganic byproducts.
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the reactants and their functional groups.
The reactants are 1 -bromo- 1 -chloro- 2,2 -dimethylpropane (a tertiary halide) and hydroxide ion (a strong nucleophile).
Step 2: Understand the reaction mechanism.
The reaction follows an SN^2 mechanism, where the nucleophile (hydroxide ion) attacks the tertiary carbon from the backside, leading to inversion of configuration.
Final Answer
The major product of the reaction is 2 -hydroxy- 2,2 -dimethylpropane, represented as: \begin{array}{c} \ce{H} \ \ce{|} \ \ce{C}- \begin{array}{c} \ce{H} \ \ce{|} \ \ce{C}- \begin{array}{c} \ce{OH} \ \ce{|} \ \ce{H} \end{array} \ \ce{|} \ \ce{CH^3} \end{array} \end{array} with the hydroxyl group (OH) on the same plane as the methyl group (CH3) and the other two methyl groups in a perpendicular plane.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students