QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
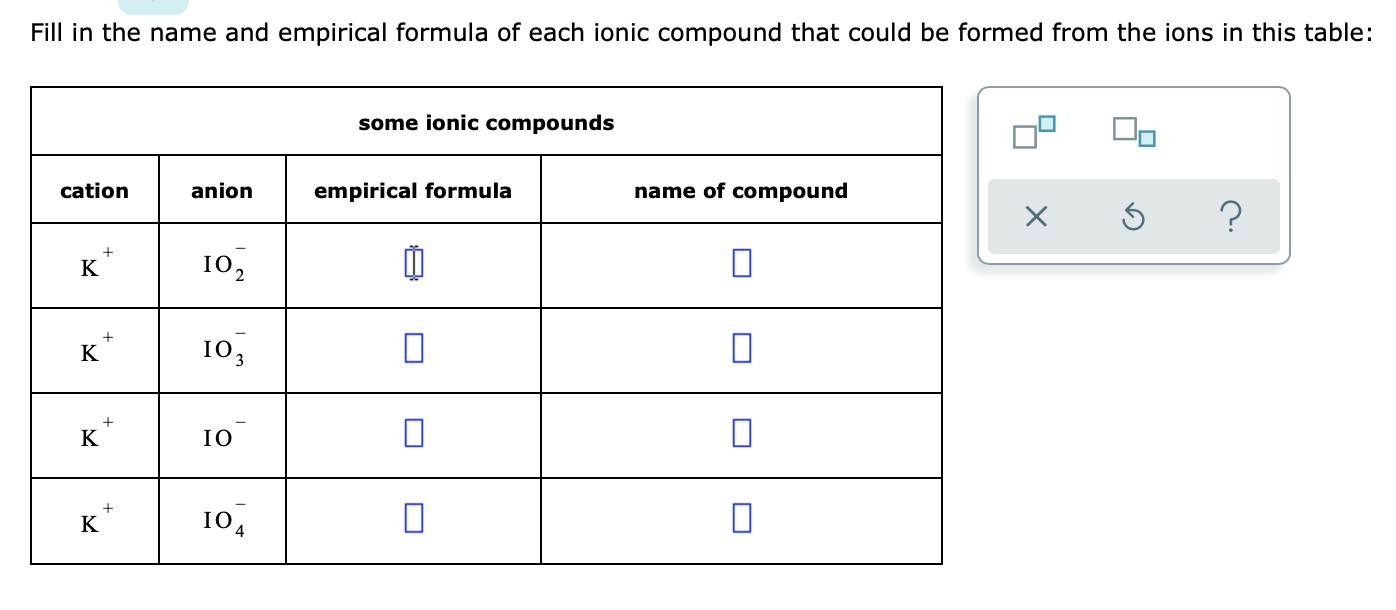
Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table:
| **s** | **some ionic compounds** | **empirical formula** | **name of compound** |
| --- | --- | --- | --- |
| cation | anion | | |
| K⁺ | 1O₂⁻ | | |
| K⁺ | 1O₃⁻ | | |
| K⁺ | 1O⁻ | | |
| K⁺ | 1O⁻ | | |
| K⁺ | 1O₄⁻ | | |
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the cation and anion in the first row.
The cation is K+ and the anion is O^2 -.
Step 2: Write the empirical formula.
Since the charges on the ions are + 1 for K and - 2 for O^2 -, the compound must have one K for every O^2 - in order to balance the charges. Therefore, the empirical formula is KO^2.
Final Answer
| **cation** | **anion** | **empirical formula** | **name of compound** | | --- | --- | --- | --- | | K+ | 1O₂⁻ | KO^2 | potassium peroxide | | K+ | 1O₃⁻ | KO^3 | potassium ozone | | K+ | 1O⁻ | K^2O | potassium oxide | | K+ | 1O⁻ | K^2O | potassium oxide | | K+ | 1O₄⁻ | K^2O^4 | potassium superoxide |
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students