QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
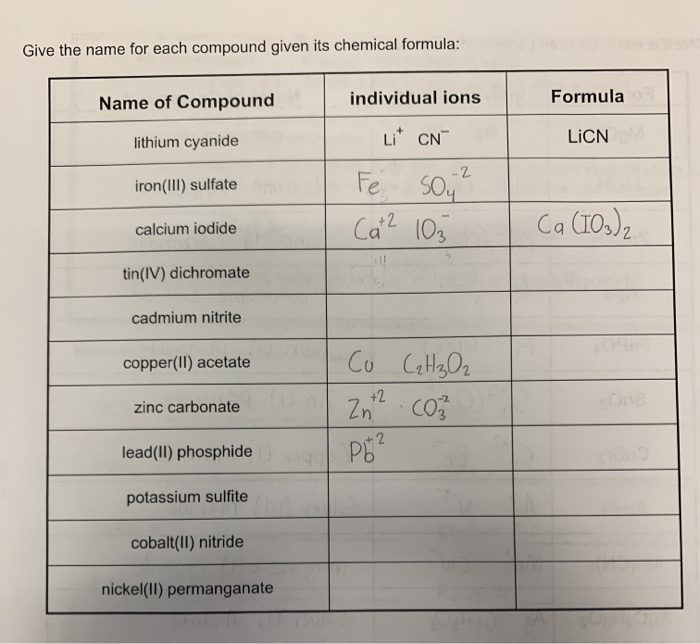
Give the name for each compound given its chemical formula:
| Name of Compound | individual ions | Formula |
| --- | --- | --- |
| lithium cyanide | Li^+ \cdot CN^- | LiCN |
| iron(III) sulfate | Fe^{3 + 4 + 5 + 6 + 7 + 8 + 9 + 10 + 11 + 12 + 13 + 14 + 15 + 16 + 17 + 18 + 19 + 20 + 21 + 22 + 23 + 24 + 25 + 26 + 27 + 28 + 29 + 30 + 31 + 32 + 33 + 34 + 35 + 36 + 37 + 38 + 39 + 40 + 41 + 42 + 43 + 44 + 45 + 46 + 47 + 48 + 49 + 50 + 51 + 52 + 53 + 54 + 55 + 56 + 57 + 58 + 59 + 60 + 61 + 62 + 63 |
| calcium iodide | Ca^{2 +} | Ca(IO3)2 |
| tin(IV) dichromate | | |
| cadmium nitrite | | |
| copper(II) acetate | Cu^{2 +} | CuO^2 |
| zinc carbonate | Zn^{2 +} | ZnO^2 |
| lead(II) phosphide | Pb^{2 +} | PbO^2 |
| potassium sulfite | | |
| cobalt(II) nitride | | |
| nickel(II) permanganate | | |
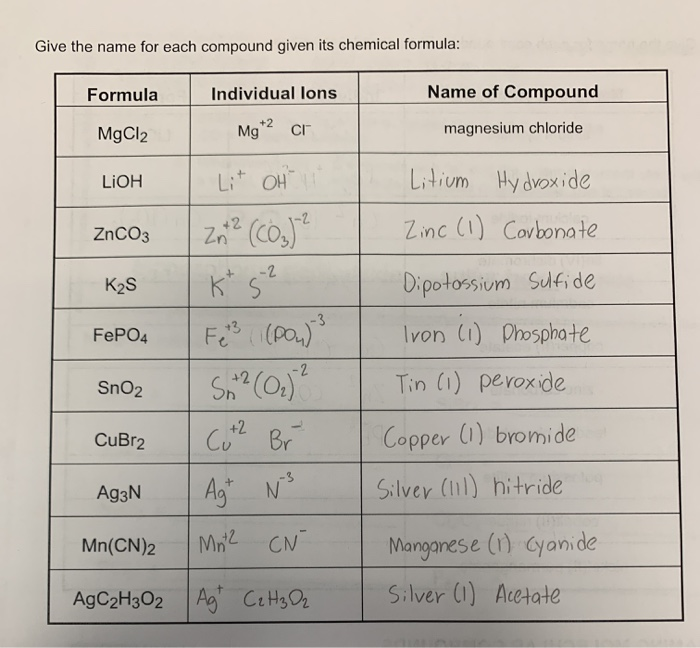
Give the name for each compound given its chemical formula:
| Formula | Individual Ions | Name of Compound |
| --- | --- | --- |
| MgCl^2 | Mg+ 2
Cl- | magnesium chloride |
| LiOH | Li+
OH- | Litium Hydroxide |
| ZnCO^3 | Zn+ 2
(CO3)2 - | Zinc (I) Carbonate |
| K^2S | K+
S^2 - | Dipotassium Sulfide |
| FePO^4 | Fe+ 3
(PO4)3 - | Iron (I) Phosphate |
| SnO^2 | Sn+ 2
(Cl2)2 - | Tin (I) Peroxide |
| CuBr^2 | Cu+ 2
Br- | Copper (I) Bromide |
| Ag^3N | Ag+
N^3 - | Silver (III) Nitride |
| Mn(CN)2 | Mn^2
CN- | Manganese (I) Cyanide |
| AgC^2H^3O^2 | Ag+
C^2H^3O^2 | Silver (I) Acetate |
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Determine the name for Iron(2$) sulfate, LiCN, Ca(2$)2, and CuO^2.
The name for LiCN is lithium cyanide, as given in the table. The formula Fe^{3 +}^{4 + 5 + 6 + 7 + 8 + 9 + 10 + 11 + 12 + 13 + 14 + 15 + 16 + 17 + 18 + 19 + 20 + 21 + 22 + 23 + 24 + 25 + 26 + 27 + 28 + 29 + 30 + 31 + 32 + 33 + 34 + 35 + 36 + 37 + 38 + 39 + 40 + 41 + 42 + 43 + 44 + 45 + 46 + 47 + 48 + 49 + 50 + 51 + 52 + 53 + 54 + 55 + 56 + 57 + 58 + 59 + 60 + 61 + 62 + 63} represents iron(2$) sulfate, as the iron has a + 3 charge. The name for Ca(2$)2 is calcium iodate, as the calcium has a + 2 charge. The formula for copper(2$) acetate is CuO^2, as the copper has a + 2 charge.
Step 2: Determine the name for Sn(2$)2, PbO^2, K^2S, ZnO^2, Ag^3N, Mn(2$)2, AgC^2H^3O^2, FePO^4, MgCl^2, LiOH, and Ni(2$)2.
The name for Sn(2$)2 is tin(2$) dichromate, as the tin has a + 4 charge. The name for PbO^2 is lead(2$) phosphide, as the lead has a + 2 charge. The name for K^2S is dipotassium sulfide, as the potassium has a + 1 charge. The name for ZnO^2 is zinc(2$) carbonate, as the zinc has a + 2 charge. The name for Ag^3N is silver(2$) nitride, as the silver has a + 1 charge. The name for Mn(2$)2 is manganese(2$) cyanide, as the manganese has a + 2 charge. The name for AgC^2H^3O^2 is silver(2$) acetate, as the silver has a + 1 charge. The name for FePO^4 is iron(2$) phosphate, as the iron has a + 3 charge. The name for MgCl^2 is magnesium chloride, as the magnesium has a + 2 charge. The name for LiOH is lithium hydroxide, as the lithium has a + 1 charge. The name for Ni(2$)2 is nickel(2$) nitrate, as the nickel has a + 2 charge.
Final Answer
| Name of Compound | Individual Ions | Formula | | --- | --- | --- | | Lithium cyanide | Li+, CN- | LiCN | | Iron(2$) sulfate | Fe^3 +, (SO4)2 - | Fe^2(SO4)3 | | Calcium iodide | Ca^2 +, (IO3)2 - | Ca(2$)2 | | Tin(2$) dichromate | Sn^4 +, (Cr^2O7)2 - | Sn(2$)2 | | Cadmium nitrite | Cd^2 +, (NO2)2 - | Cd(2$)2 | | Copper(2$) acetate | Cu^2 +, (C^2H^3O2)2 - | Cu(2$)2 | | Zinc carbonate | Zn^2 +, (CO3)2 - | ZnCO^3 | | Lead(2$) phosphide | Pb^2 +, (P)3 - | Pb^3P^2 | | Potassium sulfite | K+, (SO3)2 - | K^2SO^3 | | Cobalt(2$) nitride | Co^2 +, N^3 - | CoN | | Nickel(2$) permanganate | Ni^2 +, (MnO4)2 - | Ni(2$)2 | | Magnesium chloride | Mg^2 +, Cl- | MgCl^2 | | Lithium hydroxide | Li+, OH- | LiOH | | Nickel(2$) nitrate | Ni^2 +, (NO3)2 - | Ni(2$)2 |
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students