QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
# Identify the conjugate acid for each base:
- Conjugate acid of $\mathrm{HSO}_{4}^{-}$
- Conjugate acid of $\mathrm{SO}_{4}^{2 -}$
- Conjugate acid of $\mathrm{NH}_{3}$
Attachments
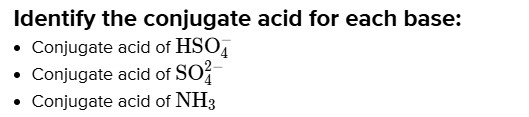
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I'll solve this chemistry problem step by step, following the specified LaTeX formatting guidelines:
Step 2: Understanding Conjugate Acids
A conjugate acid is formed when a base accepts a proton (H⁺). To find the conjugate acid, we add a proton (H⁺) to the base.
Final Answer
- Conjugate acid of \mathrm{HSO}_{4}^{-}: \mathrm{H}_{2}\mathrm{SO}_{4} - Conjugate acid of \mathrm{SO}_{4}^{2 -}: \mathrm{HSO}_{4}^{-} - Conjugate acid of \mathrm{NH}_{3}: \mathrm{NH}_{4}^{+}
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students