QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
Identify the element with the electron configuration: 1s^22 s^22 p^63 s^23 p^64 s^23 d^{10}4p^6.
Attachments
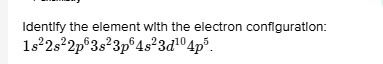
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the period and group of the element
The electron configuration provided shows that all orbitals up to and including 4p are filled. This corresponds to the completion of the fourth energy level. In the periodic table, the fourth energy level corresponds to the fourth period. To identify the group, we need to look at the outermost electrons, which are in the 4s and 3d orbitals. The 3d electrons are in a lower energy state than the 4s electrons, so we consider the 4s electrons first. The 4s orbital can hold up to 2 electrons, and here it has 2 electrons. Therefore, this element is a transition metal, which are located in the middle of the periodic table in columns 3 - 12 (excluding group 12).
Step 2: Identify the atomic number
The electron configuration $$1s^22s^22p^63s^23p^64s^23d^{10}4p^6$$ has a total of 54 electrons.
The number of electrons in an atom is equal to its atomic number. Since we have accounted for all the electrons in the given electron configuration, we can determine the atomic number by counting them. Therefore, the atomic number of the element is 54.
Final Answer
The element with the electron configuration 1s^22 s^22 p^63 s^23 p^64 s^23 d^{10}4p^6 is Xenon (Xe).
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students