QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
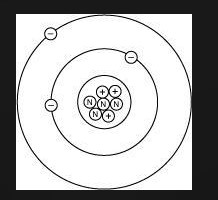
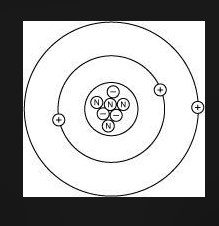
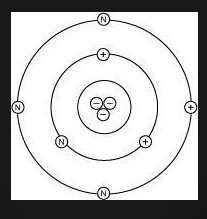
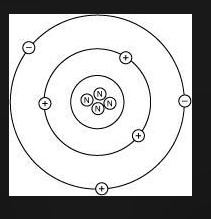
Lithium has 3 electrons, 3 protons, and 4 neutrons. Which diagram shows the correct position of electrons, neutrons, and protons in a Lithium atom?
CHOOSE Either picture 1 2 3 or 4
.
(1)
.
\begin{aligned}
& \text { 今 } \\
& \text { 今 }
\end{aligned}
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I'll solve this step by step, focusing on the atomic structure of Lithium.
Step 2: Understand the Atomic Composition
- Lithium has: * 3 protons (atomic number) * 3 electrons (to balance the protons) * 4 neutrons (given in the problem statement)
Final Answer
Picture 4 shows the correct position of electrons, neutrons, and protons in a Lithium atom.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students