QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
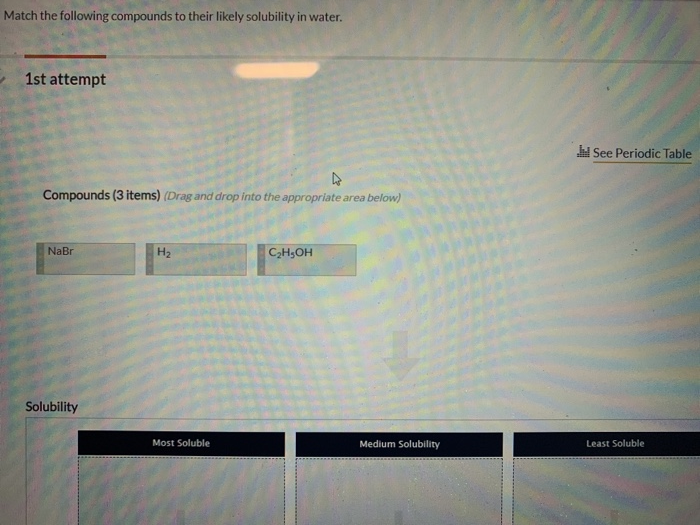
Match the following compounds to their likely solubility in water.
# 1st attempt
Compounds (3 items) (Drag and drop into the appropriate area below)
NaBr
$\mathrm{H}_{2}$
$\mathrm{C}_{3} \mathrm{H}_{5} \mathrm{OH}$
Solubility
Most Soluble
Medium Solubility
Least Soluble
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1**Step 1:** First, let's discuss the solubility rules for ionic compounds and covalent compounds in water.
Covalent compounds, like $\mathrm{H}_{2}$, are generally insoluble in water.
Ionic compounds, like NaBr, generally dissolve well in water. **Step 2:** Now, let's match the compounds to their likely solubility in water based on the solubility rules.
Step 2
NaBr: Ionic compound, so it should have high solubility in water. Explanation: Sodium bromide (NaBr) is an ionic compound that consists of sodium ions (Na+) and bromide ions (Br-). Ionic compounds usually dissociate into ions when they dissolve in water. Since NaBr dissociates completely, it has high solubility in water. **
Final Answer
2. $\mathrm{H}_{2}$: Covalent compound, so it should have low solubility in water. Explanation: Hydrogen gas ($\mathrm{H}_{2}$) is a homonuclear diatomic molecule and a covalent compound. Covalent compounds typically do not dissolve in water because they do not dissociate into ions. Therefore, $\mathrm{H}_{2}$ has low solubility in water. 3. $\mathrm{C}_{3} \mathrm{H}_{5} \mathrm{OH}$: Alcohol, so it should have medium solubility in water. Explanation: Propanol ($\mathrm{C}_{3} \mathrm{H}_{5} \mathrm{OH}$) is an alcohol, which contains both polar and nonpolar components. Alcohols have limited solubility in water due to the hydrophobic nature of their alkyl chain. However, the polar hydroxyl group (-OH) allows alcohols to form hydrogen bonds with water molecules. Therefore, propanol has medium solubility in water.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students