QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
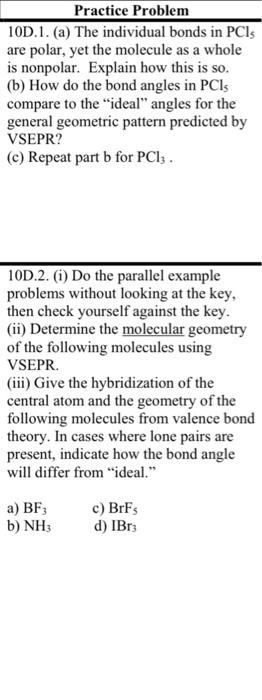
# Practice Problem
10D.1. (a) The individual bonds in $\mathrm{PCl}_{5}$ are polar, yet the molecule as a whole is nonpolar. Explain how this is so.
(b) How do the bond angles in $\mathrm{PCl}_{5}$ compare to the "ideal" angles for the general geometric pattern predicted by VSEPR?
(c) Repeat part b for $\mathrm{PCl}_{3}$.
10D.2. (i) Do the parallel example problems without looking at the key, then check yourself against the key.
(ii) Determine the molecular geometry of the following molecules using VSEPR.
(iii) Give the hybridization of the central atom and the geometry of the following molecules from valence bond theory. In cases where lone pairs are present, indicate how the bond angle will differ from "ideal."
a) $\mathrm{BF}_{3}$
c) $\mathrm{BrF}_{5}$
b) $\mathrm{NH}_{3}$
d) $\mathrm{IBr}_{3}$
10D.3. Predict the electron-domain geometry and the hybridization of the central atom in
(a) $\mathrm{SO}_{3}{ }^{2 -}$
(b) $\mathrm{SF}_{6}$
10D.4. In each of the following pairs, which ion or molecule has the smaller bond angle(s)?
a) $\mathrm{SF}_{6}$ or $\mathrm{H}_{2} \mathrm{~S}$
b) $\mathrm{BF}_{3}$ or $\mathrm{NH}_{4}^{+}$
c) $\mathrm{H}_{2} \mathrm{O}$ or $\mathrm{NH}_{3}$
Attachments

6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I'll provide detailed solutions for each part of the practice problems.
Since some of the questions involve conceptual explanations, I will format the LaTeX syntax where applicable.
Step 2(a) The individual bonds in $\mathrm{PCl}_{5}$ are polar due to the difference in electronegativity between phosphorus (P) and chlorine (Cl).
Experimentally, the $\mathrm{P-Cl}$ bond angle in $\mathrm{PCl}_{3}$ is about $100.3\degree$, which is a relatively small deviation from the ideal angle.
However, the molecule as a whole is nonpolar because the polar bonds cancel each other out. The vector sum of all the bond dipoles is zero, resulting in a nonpolar molecule.
Final Answer
However, $\mathrm{NH}_{3}$ has a smaller deviation from the ideal bond angle compared to $\mathrm{H}_{2} \mathrm{O}$.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students