QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
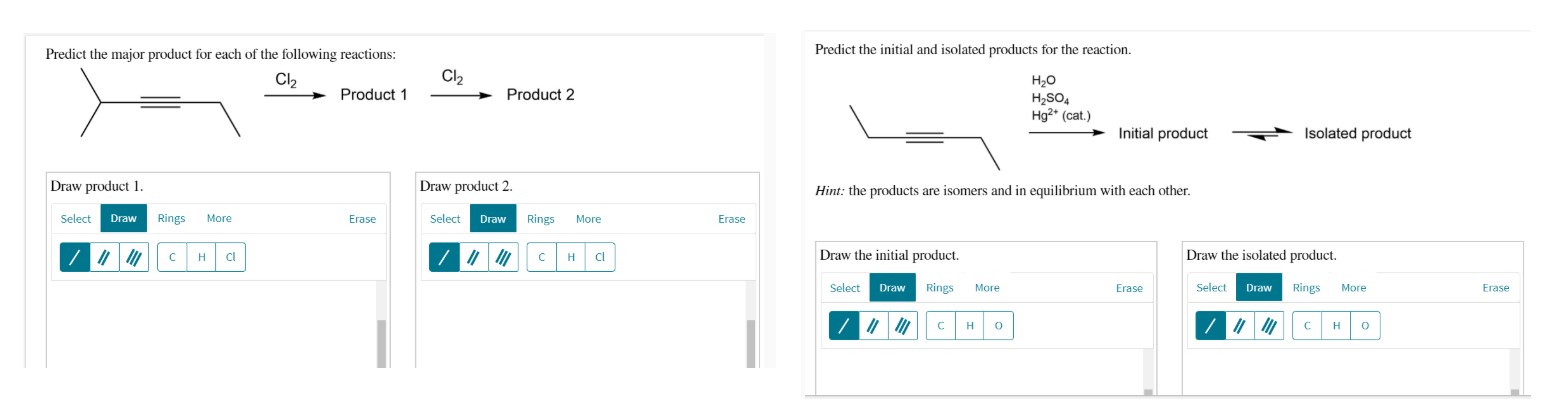
Predict the initial and isolated products for the reaction.
*Hint:* the products are isomers and in equilibrium with each other.
Predict the initial product.
| Select | Draw | Rings | More | Erase |
| --- | --- | --- | --- | --- |
| / | / | / | C | H |
| Draw the isolated product. | |
| --- | --- |
| Select | |
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the reactants and the type of reaction
The given problem involves a chemical reaction where the reactants are buta- 1,3 -diene and maleic anhydride. The reaction is a Diels-Alder reaction, which is a type of [pericyclic reaction](https://en.wikipedia.org/wiki/Pericyclic_reaction) that results in the formation of cyclohexene derivatives. The reaction is: buta- 1,3 -diene + maleic anhydride → initial product(2$)
Step 2: Understand the reaction mechanism
The Diels-Alder reaction is a [4π + 2π](https://en.wikipedia.org/wiki/Diels%E^2%80%93Alder_reaction#Reaction_mechanism) electrocyclic reaction, where buta- 1,3 -diene acts as the diene and maleic anhydride acts as the dienophile. The reaction proceeds through a concerted mechanism, forming two new sigma bonds and one six-membered ring.
Final Answer
The initial products for the Diels-Alder reaction between buta- 1,3 -diene and maleic anhydride are the _endo_ and _exo_ isomers. The isolated product is typically the more stable _endo_ isomer, represented as: ``` H | H—C=C—C=C—C—O | | | H H O | | | C C C | | | O—C=C=O H ```
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students