QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
# What is the conjugate acid of $\mathrm{HCO}_{3}^{-}$?
A. $\mathrm{OH}^{-}$
B. $\mathrm{H}_{2} \mathrm{CO}_{3}$
C. $\mathrm{CO}_{3}^{2 -}$
D. $\mathrm{H}_{2} \mathrm{O}$
E. $\mathrm{H}_{3} \mathrm{O}^{+}$
Attachments
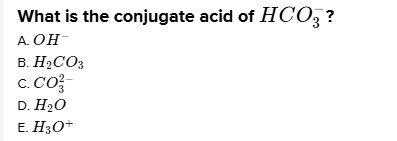
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the acid and its charge
The given acid is $\mathrm{HCO}_{3}^{-}$.
It has a charge of - 1.
Step 2: Understand conjugate acid-base pairs
A conjugate acid-base pair differs by only one proton (H+). The conjugate base of an acid is formed when the acid donates a proton (gains a hydrogen ion), and the conjugate acid of a base is formed when the base accepts a proton (loses a hydrogen ion).
Final Answer
The conjugate acid of $\mathrm{HCO}_{3}^{-}$ is $\mathrm{H}_{2} \mathrm{CO}_{3}$.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students